Synthesis and Application of Trehalose Materials
- PMID: 35911465
- PMCID: PMC9327084
- DOI: 10.1021/jacsau.2c00309
Synthesis and Application of Trehalose Materials
Abstract
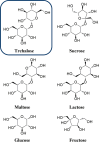
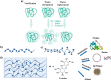
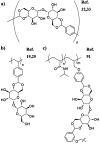
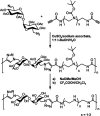
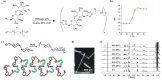
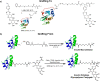
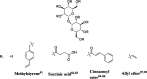
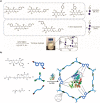
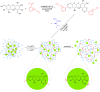
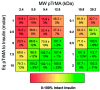
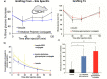
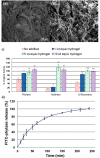
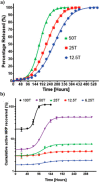
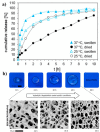
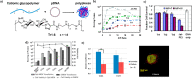
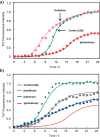
Trehalose is a naturally occurring, nonreducing disaccharide that is widely used in the biopharmaceutical, food, and cosmetic industries due to its stabilizing and cryoprotective properties. Over the years, scientists have developed methodologies to synthesize linear polymers with trehalose units either in the polymer backbone or as pendant groups. These macromolecules provide unique properties and characteristics, which often outperform trehalose itself. Additionally, numerous reports have focused on the synthesis and formulation of materials based on trehalose, such as nanoparticles, hydrogels, and thermoset networks. Among many applications, these polymers and materials have been used as protein stabilizers, as gene delivery systems, and to prevent amyloid aggregate formation. In this Perspective, recent developments in the synthesis and application of trehalose-based linear polymers, hydrogels, and nanomaterials are discussed, with a focus on utilization in the biomedical field.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Liu Q.; Schmidt R. K.; Teo B.; Karplus P. A.; Brady J. W. Molecular Dynamics Studies of the Hydration of α,α-Trehalose. J. Am. Chem. Soc. 1997, 119 (33), 7851–7862. 10.1021/ja970798v. - DOI
-
- Donnamaria M. C.; Howard E. I.; Grigera J. R. Interaction of Water with α,α-Trehalose in Solution: Molecular Dynamics Simulation Approach. J. Chem. Soc., Faraday Trans. 1994, 90 (18), 2731–2735. 10.1039/FT9949002731. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
