Mitigation of MAFLD in High Fat-High Sucrose-Fructose Fed Mice by a Combination of Genistein Consumption and Exercise Training
- PMID: 35911503
- PMCID: PMC9329575
- DOI: 10.2147/DMSO.S358256
Mitigation of MAFLD in High Fat-High Sucrose-Fructose Fed Mice by a Combination of Genistein Consumption and Exercise Training
Abstract
Purpose: Metabolic dysfunction-associated fatty liver disease (MAFLD) is fueled by escalations in both sedentary behavior and caloric intake and is noted in obese type 2 diabetic (T2DM) patients. This study aimed to examine the effects of exercise and the phytoestrogen genistein in mice fed a high fat (60% fat) high sugar (55% fructose with 45% sucrose), HFHS diet.
Methods: Male C57BL/6J mice were assigned to five groups: HFHS, HFHS with genistein (600 mg/kg diet, HFHS+Gen), HFHS with moderate exercise (HFHS+Ex), and HFHS with combined genistein and moderate exercise (HFHS-Gen+Ex). Control lean mice were fed standard chow and water. Exercise consisted of 30-minute sessions of treadmill running five days/week for the 12-week study duration. Body weight was assessed weekly. Liver, kidney, fecal pellets and serum were extracted at the end of the study and maintained at -80°C.
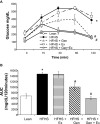
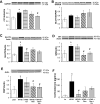
Results: After 12 weeks of treatment, mice in the HFHS group had the highest hepatic lipid content. Plasma levels of glucose, insulin, leptin, cholesterol, amylin, and total fat content were significantly elevated in HFHS mice compared to control mice. HFHS feeding increased protein expression of carnitine palmitoyltransferase 1b (CPT-1b isoform) in gastrocnemius, CPT1a, glucose transporter protein 2 (GLUT2), glucocorticoid receptor (GR), and fructose 1,6-bisphosphate 1 (FBP1) expression in liver. Exercise alone had minor effects on these metabolic abnormalities. Genistein alone resulted in improvements in body weight, fat content, amylin, insulin sensitivity, and liver histopathology, GR, FBP1, and acetyl-CoA carboxylase 1 (ACC1). Combination treatment resulted in additional metabolic improvements, including reductions in hepatic lipid content and lipid area, alanine transferase activity, CPT1b, and CPT1a.
Conclusion: Our results indicate that a HFHS diet is obesogenic, inducing metabolic perturbations consistent with T2DM and MAFLD. Genistein alone and genistein combined with moderate intensity exercise were effective in reducing MAFLD and the aberrations induced by chronic HFHS feeding.
Keywords: Soy isoflavone; diabetes; exercise; genistein; hepatic steatosis; western diet.
© 2022 St Aubin et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Influence of Exercise and Genistein to Mitigate the Deleterious Effects of High-Fat High-Sugar Diet on Alzheimer's Disease-Related Markers in Male Mice.Int J Mol Sci. 2024 Aug 20;25(16):9019. doi: 10.3390/ijms25169019. Int J Mol Sci. 2024. PMID: 39201705 Free PMC article.
-
Bone Strength Is Improved with Genistein Treatment in Mice with Diet-Induced Obesity.Curr Dev Nutr. 2019 Oct 23;3(11):nzz121. doi: 10.1093/cdn/nzz121. eCollection 2019 Nov. Curr Dev Nutr. 2019. PMID: 31750414 Free PMC article.
-
Effects of Exercise Training on Renal Carnitine Biosynthesis and Uptake in the High-Fat and High-Sugar-Fed Mouse.Molecules. 2020 Apr 30;25(9):2100. doi: 10.3390/molecules25092100. Molecules. 2020. PMID: 32365864 Free PMC article.
-
Fecal microbiota transplantation from high caloric-fed donors alters glucose metabolism in recipient mice, independently of adiposity or exercise status.Am J Physiol Endocrinol Metab. 2020 Jul 1;319(1):E203-E216. doi: 10.1152/ajpendo.00037.2020. Epub 2020 Jun 9. Am J Physiol Endocrinol Metab. 2020. PMID: 32516027
-
The metabolic associated fatty liver disease responses of lifestyle changes using diet and exercise.Pak J Med Sci. 2023 Nov-Dec;39(6):1875-1882. doi: 10.12669/pjms.39.6.7990. Pak J Med Sci. 2023. PMID: 37936729 Free PMC article. Review.
Cited by
-
Alfalfa Xeno-miR168b Target CPT1A to Regulate Milk Fat Synthesis in Bovine Mammary Epithelial Cells.Metabolites. 2023 Jan 3;13(1):76. doi: 10.3390/metabo13010076. Metabolites. 2023. PMID: 36677001 Free PMC article.
-
The effect of soy isoflavones supplementation on metabolic status in patients with non-alcoholic fatty liver disease: a randomized placebo controlled clinical trial.BMC Public Health. 2024 May 21;24(1):1362. doi: 10.1186/s12889-024-18812-3. BMC Public Health. 2024. PMID: 38773414 Free PMC article. Clinical Trial.
-
Effects of Genistein on Lipid Metabolism, Antioxidant Activity, and Immunity of Common Carp (Cyprinus carpio L.) Fed with High-Carbohydrate and High-Fat Diets.Aquac Nutr. 2023 Mar 31;2023:9555855. doi: 10.1155/2023/9555855. eCollection 2023. Aquac Nutr. 2023. PMID: 37034827 Free PMC article.
-
Influence of Exercise and Genistein to Mitigate the Deleterious Effects of High-Fat High-Sugar Diet on Alzheimer's Disease-Related Markers in Male Mice.Int J Mol Sci. 2024 Aug 20;25(16):9019. doi: 10.3390/ijms25169019. Int J Mol Sci. 2024. PMID: 39201705 Free PMC article.
-
Genistein Suppresses IL-6 and MMP-13 to Attenuate Osteoarthritis in Obese Diabetic Mice.Metabolites. 2023 Sep 14;13(9):1014. doi: 10.3390/metabo13091014. Metabolites. 2023. PMID: 37755294 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

