Low α2-Plasmin Inhibitor Antigen Levels on Admission Are Associated With More Severe Stroke and Unfavorable Outcomes in Acute Ischemic Stroke Patients Treated With Intravenous Thrombolysis
- PMID: 35911531
- PMCID: PMC9334909
- DOI: 10.3389/fcvm.2022.901286
Low α2-Plasmin Inhibitor Antigen Levels on Admission Are Associated With More Severe Stroke and Unfavorable Outcomes in Acute Ischemic Stroke Patients Treated With Intravenous Thrombolysis
Abstract
Background: Intravenous administration of recombinant tissue plasminogen activator (rt-PA) fails to succeed in a subset of acute ischemic stroke (AIS) patients, while in approximately 6-8% of cases intracerebral hemorrhage (ICH) occurs as side effect.
Objective: Here, we aimed to investigate α2-plasmin inhibitor (α2-PI) levels during thrombolysis and to find out whether they predict therapy outcomes in AIS patients.
Patients/methods: In this prospective, observational study, blood samples of 421 AIS patients, all undergoing i.v. thrombolysis by rt-PA within 4.5 h of their symptom onset, were taken before and 24 h after thrombolysis. In a subset of patients (n = 131), blood was also obtained immediately post-lysis. α2-PI activity and antigen levels were measured by chromogenic assay and an in-house ELISA detecting all forms of α2-PI. α2-PI Arg6Trp polymorphism was identified in all patients. Stroke severity was determined by NIHSS on admission and day 7. Therapy-associated ICH was classified according to ECASSII. Long-term outcomes were defined at 3 months post-event by the modified Rankin Scale (mRS).
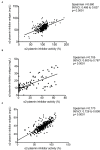
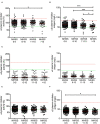
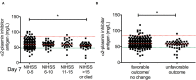
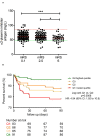
Results: Median α2-PI activity and antigen levels showed a significant drop immediately post-lysis and increased to subnormal levels at 24 h post-event. Admission α2-PI levels showed a significant negative stepwise association with stroke severity. Patients with favorable long-term outcomes (mRS 0-1) had significantly higher admission α2-PI antigen levels (median:61.6 [IQR:55.9-70.5] mg/L) as compared to patients with poor outcomes (mRS 2-5: median:59.7 [IQR:54.5-69.1] and mRS 6: median:56.0 [IQR:48.5-61.0] mg/L, p < 0.001). In a Kaplan-Meier survival analysis, patients with an α2-PI antigen in the highest quartile on admission showed significantly better long-term survival as compared to those with α2-PI antigen in the lowest quartile (HR: 4.54; 95%CI:1.92-10.8, p < 0.001); however, in a multivariate analysis, a low admission α2-PI antigen did not prove to be an independent risk factor of poor long-term outcomes. In patients with therapy-related ICH (n = 34), admission α2-PI antigen levels were significantly, but only marginally, lower as compared to those without hemorrhage.
Conclusions: Low α2-PI antigen levels on admission were associated with more severe strokes and poor long-term outcomes in this cohort. Our results suggest that in case of more severe strokes, α2-PI may be involved in the limited efficacy of rt-PA thrombolysis.
Keywords: acute ischemic stroke; fibrinolysis; intracerebral hemorrhage (ICH); outcome; recombinant tissue plasminogen activator (rt-PA); thrombolysis; α2-plasmin inhibitor.
Copyright © 2022 Székely, Orbán-Kálmándi, Szegedi, Katona, Baráth, Czuriga-Kovács, Lóczi, Vasas, Fekete, Fekete, Berényi, Oláh, Csiba and Bagoly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–e110. 10.1161/STR.0000000000000158 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

