Monte Carlo Simulation and Reconstruction: Assessment of Myocardial Perfusion Imaging of Tracer Dynamics With Cardiac Motion Due to Deformation and Respiration Using Gamma Camera With Continuous Acquisition
- PMID: 35911544
- PMCID: PMC9326051
- DOI: 10.3389/fcvm.2022.871967
Monte Carlo Simulation and Reconstruction: Assessment of Myocardial Perfusion Imaging of Tracer Dynamics With Cardiac Motion Due to Deformation and Respiration Using Gamma Camera With Continuous Acquisition
Abstract
Purpose: Myocardial perfusion imaging (MPI) with single photon emission computed tomography (SPECT) is routinely used for stress testing in nuclear medicine. Recently, our group extended its potential going from 3D visual qualitative image analysis to 4D spatiotemporal reconstruction of dynamically acquired data to capture the time variation of the radiotracer concentration and the estimated myocardial blood flow (MBF) and coronary flow reserve (CFR). However, the quality of reconstructed image is compromised due to cardiac deformation and respiration. The work presented here develops an algorithm that reconstructs the dynamic sequence of separate respiratory and cardiac phases and evaluates the algorithm with data simulated with a Monte Carlo simulation for the continuous image acquisition and processing with a slowly rotating SPECT camera.
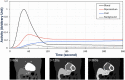
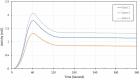
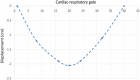
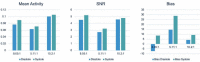
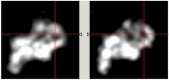
Methods: A clinically realistic Monte Carlo (MC) simulation is developed using the 4D Extended Cardiac Torso (XCAT) digital phantom with respiratory and cardiac motion to model continuous data acquisition of dynamic cardiac SPECT with slowly rotating gamma cameras by incorporating deformation and displacement of the myocardium due to cardiac and respiratory motion. We extended our previously developed 4D maximum-likelihood expectation-maximization (MLEM) reconstruction algorithm for a data set binned from a continuous list mode (LM) simulation with cardiac and respiratory information. Our spatiotemporal image reconstruction uses splines to explicitly model the temporal change of the tracer for each cardiac and respiratory gate that delineates the myocardial spatial position as the tracer washes in and out. Unlike in a fully list-mode data acquisition and reconstruction the accumulated photons are binned over a specific but very short time interval corresponding to each cardiac and respiratory gate. Reconstruction results are presented showing the dynamics of the tracer in the myocardium as it continuously deforms. These results are then compared with the conventional 4D spatiotemporal reconstruction method that models only the temporal changes of the tracer activity. Mean Stabilized Activity (MSA), signal to noise ratio (SNR) and Bias for the myocardium activities for three different target-to-background ratios (TBRs) are evaluated. Dynamic quantitative indices such as wash-in (K1) and wash-out (k2) rates at each gate were also estimated.
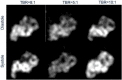
Results: The MSA and SNR are higher with higher TBRs while biases were improved with higher TBRs to less than 10%. The correlation between exhalation-inhalation sequence with the ground truth during respiratory cycle was excellent. Our reconstruction method showed better resolved myocardial walls during diastole to systole as compared to the ungated 4D image. Estimated values of K1 and k2 were also consistent with the ground truth.
Conclusion: The continuous image acquisition for dynamic scan using conventional two-head gamma cameras can provide valuable information for MPI. Our study demonstrated the viability of using a continuous image acquisition method on a widely used clinical two-head SPECT system. Our reconstruction method showed better resolved myocardial walls during diastole to systole as compared to the ungated 4D image. Precise implementation of reconstruction algorithms, better segmentation techniques by generating images of different tissue types and background activity would improve the feasibility of the method in real clinical environment.
Keywords: cardiac motion; continuous acquisition; dynamic SPECT; motion correction; myocardial perfusion imaging.
Copyright © 2022 Huh, Shrestha, Gullberg and Seo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
Image reconstruction in higher dimensions: myocardial perfusion imaging of tracer dynamics with cardiac motion due to deformation and respiration.Phys Med Biol. 2015 Nov 7;60(21):8275-301. doi: 10.1088/0031-9155/60/21/8275. Epub 2015 Oct 9. Phys Med Biol. 2015. PMID: 26450115 Free PMC article.
-
Investigation of dynamic SPECT measurements of the arterial input function in human subjects using simulation, phantom and human studies.Phys Med Biol. 2012 Jan 21;57(2):375-93. doi: 10.1088/0031-9155/57/2/375. Epub 2011 Dec 14. Phys Med Biol. 2012. PMID: 22170801 Free PMC article.
-
4D numerical observer for lesion detection in respiratory-gated PET.Med Phys. 2014 Oct;41(10):102504. doi: 10.1118/1.4895975. Med Phys. 2014. PMID: 25281979 Free PMC article.
-
PET and SPECT Tracers for Myocardial Perfusion Imaging.Semin Nucl Med. 2020 May;50(3):208-218. doi: 10.1053/j.semnuclmed.2020.02.016. Epub 2020 Mar 13. Semin Nucl Med. 2020. PMID: 32284107 Review.
-
Dynamic single photon emission computed tomography--basic principles and cardiac applications.Phys Med Biol. 2010 Oct 21;55(20):R111-91. doi: 10.1088/0031-9155/55/20/R01. Epub 2010 Sep 22. Phys Med Biol. 2010. PMID: 20858925 Free PMC article. Review.
Cited by
-
Multi-pinhole collimator design in different numbers of projections for brain SPECT.Front Med (Lausanne). 2023 Sep 28;10:1211726. doi: 10.3389/fmed.2023.1211726. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37841005 Free PMC article.
References
-
- Ueshima K, Yamashina A, Usami S, Yasuno S, Nishiyama O, Yamazaki T, et al. Prognostic value of myocardial perfusion SPECT images in combination with the maximal heart rate at exercise testing in Japanese patients with suspected ischemic heart disease: a sub-analysis of J-ACCESS. Ann Nucl Med. (2009) 23:849–54. 10.1007/s12149-009-0315-8 - DOI - PubMed

