TAX and HBZ: hFc Ɣ 1 proteins as targets for passive immunotherapy
- PMID: 35911645
- PMCID: PMC9282740
- DOI: 10.22038/IJBMS.2022.64787.14266
TAX and HBZ: hFc Ɣ 1 proteins as targets for passive immunotherapy
Abstract
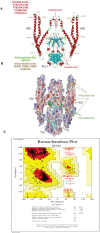
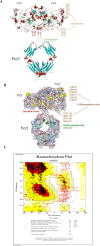
Objectives: Human T leukemia virus type one (HTLV-1) causes two life-threatening diseases in around five percent of infected subjects, a T cell malignancy and a neurodegenerative disease. TAX and HBZ are the main virulence agents implicated in the manifestation of HTLV-1-associated diseases. Therefore, this study aims to produce these HTLV-1 factors as recombinant Fc fusion proteins to study the structures, their immunogenic properties as vaccines, and their capability to produce specific neutralization antibodies.
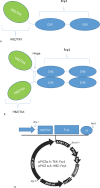
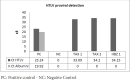
Materials and methods: TAX and HBZ sequences were chosen from the NCBI-nucleotide database, then designed as human Fc chimers and cloned into Pichia pastoris. Produced proteins were purified by HiTrap affinity chromatography and subcutaneously injected into rabbits. Rabbit Abs were purified by batch chromatography, and their neutralization activities for the HTLV-1-infected MT-2 cell line were assessed. Furthermore, the protective abilities of recombinant proteins were evaluated in Tax or HBZ immunized rabbits by MT-2 cell line inoculation and measurement of HTLV-1-proviral load.
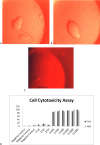
Results: Specific Abs against Tax and HBZ can eliminate 2 million MT-2 cells in 1/1000 dilution in vitro. In challenging assays, the immunization of the animals using Tax or HBZ had no protective activity as HTLV-1 PVL was still positive.
Conclusion: The result suggests that recombinant TAX and HBZ: hFcγ1 proteins can produce a proper humoral immune response. Therefore, they could be considered a passive immunotherapy source for HTLV-1-associated diseases, while total TAX and HBZ proteins are unsuitable as HTLV-1 vaccine candidates.
Keywords: ATLL; HBZ; HTLV; Immunization passive; Pichia pastoris; Recombinant proteins; TAX.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures





Similar articles
-
Evaluation of the role of TAX, HBZ, and HTLV-1 proviral load on the survival of ATLL patients.Blood Res. 2017 Jun;52(2):106-111. doi: 10.5045/br.2017.52.2.106. Epub 2017 Jun 22. Blood Res. 2017. PMID: 28698846 Free PMC article.
-
The effect of HTLV-1 virulence factors (HBZ, Tax, proviral load), HLA class I and plasma neopterin on manifestation of HTLV-1 associated myelopathy tropical spastic paraparesis.Virus Res. 2017 Jan 15;228:1-6. doi: 10.1016/j.virusres.2016.11.009. Epub 2016 Nov 12. Virus Res. 2017. PMID: 27845163
-
The Expression of Tax and HBZ Genes in Serum-Derived Extracellular Vesicles From HTLV-1 Carriers Correlates to Proviral Load and Inflammatory Markers.Front Microbiol. 2022 May 2;13:881634. doi: 10.3389/fmicb.2022.881634. eCollection 2022. Front Microbiol. 2022. PMID: 35586867 Free PMC article.
-
Role of HTLV-1 Tax and HBZ in the Pathogenesis of HAM/TSP.Front Microbiol. 2017 Dec 21;8:2563. doi: 10.3389/fmicb.2017.02563. eCollection 2017. Front Microbiol. 2017. PMID: 29312243 Free PMC article. Review.
-
NF-κB and MicroRNA Deregulation Mediated by HTLV-1 Tax and HBZ.Pathogens. 2019 Dec 10;8(4):290. doi: 10.3390/pathogens8040290. Pathogens. 2019. PMID: 31835460 Free PMC article. Review.
Cited by
-
Possible Trace of HTLV-1 Virus in Modulation of Cbl-b, ITCH, and PP2A Suppressor Genes.Int J Mol Cell Med. 2025;14(1):472-482. doi: 10.22088/IJMCM.BUMS.14.1.472. Int J Mol Cell Med. 2025. PMID: 40123588 Free PMC article.
-
Development of a Novel HTLV-1 Protease: Human Fcγ1 Recombinant Fusion Molecule in the CHO Eukaryotic Expression System.Appl Biochem Biotechnol. 2023 Mar;195(3):1862-1876. doi: 10.1007/s12010-022-04259-y. Epub 2022 Nov 18. Appl Biochem Biotechnol. 2023. PMID: 36399306 Free PMC article.
-
Human T-Lymphotropic Virus (HTLV): Epidemiology, Genetic, Pathogenesis, and Future Challenges.Viruses. 2025 May 1;17(5):664. doi: 10.3390/v17050664. Viruses. 2025. PMID: 40431676 Free PMC article. Review.
References
-
- Khan MY, Khan IN, Farman M, Al Karim S, Qadri I, Kamal MA, et al. HTLV-1 associated neurological disorders. Curr Top Med Chem. 2017;17:1320–1330. - PubMed
-
- Taylor GP. The epidemiology of HTLV-I in Europe. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;1:8–14. - PubMed
-
- Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SK, Ahmadi S, et al. High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology survey. J Clin Virol. 2011;52:172–176. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
