An ordinal severity scale for COVID-19 retrospective studies using Electronic Health Record data
- PMID: 35911666
- PMCID: PMC9278199
- DOI: 10.1093/jamiaopen/ooac066
An ordinal severity scale for COVID-19 retrospective studies using Electronic Health Record data
Abstract
Objectives: Although the World Health Organization (WHO) Clinical Progression Scale for COVID-19 is useful in prospective clinical trials, it cannot be effectively used with retrospective Electronic Health Record (EHR) datasets. Modifying the existing WHO Clinical Progression Scale, we developed an ordinal severity scale (OS) and assessed its usefulness in the analyses of COVID-19 patient outcomes using retrospective EHR data.
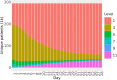
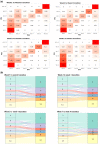
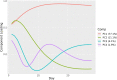
Materials and methods: An OS was developed to assign COVID-19 disease severity using the Observational Medical Outcomes Partnership common data model within the National COVID Cohort Collaborative (N3C) data enclave. We then evaluated usefulness of the developed OS using heterogenous EHR data from January 2020 to October 2021 submitted to N3C by 63 healthcare organizations across the United States. Principal component analysis (PCA) was employed to characterize changes in disease severity among patients during the 28-day period following COVID-19 diagnosis.
Results: The data set used in this analysis consists of 2 880 456 patients. PCA of the day-to-day variation in OS levels over the totality of the 28-day period revealed contrasting patterns of variation in disease severity within the first and second 14 days and illustrated the importance of evaluation over the full 28-day period.
Discussion: An OS with well-defined, robust features, based on discrete EHR data elements, is useful for assessments of COVID-19 patient outcomes, providing insights on the progression of COVID-19 disease severity over time.
Conclusions: The OS provides a framework that can facilitate better understanding of the course of acute COVID-19, informing clinical decision-making and resource allocation.
Keywords: COVID-19 ordinal scale; Electronic Health Record; N3C; National COVID Cohort Collaborative.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures



References
-
- Klann JG, Estiri H, Weber GM, et al.; Consortium for Clinical Characterization of COVID-19 by EHR (4CE) (CONSORTIA AUTHOR). Validation of an internationally derived patient severity phenotype to support COVID-19 analytics from electronic health record data. J Am Med Inform Assoc 2021; 28 (7): 1411–20. - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
