Combination Approaches to Target PD-1 Signaling in Cancer
- PMID: 35911672
- PMCID: PMC9330480
- DOI: 10.3389/fimmu.2022.927265
Combination Approaches to Target PD-1 Signaling in Cancer
Abstract
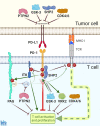
Cancer remains the second leading cause of death in the US, accounting for 25% of all deaths nationwide. Immunotherapy techniques bolster the immune cells' ability to target malignant cancer cells and have brought immense improvements in the field of cancer treatments. One important inhibitory protein in T cells, programmed cell death protein 1 (PD-1), has become an invaluable target for cancer immunotherapy. While anti-PD-1 antibody therapy is extremely successful in some patients, in others it fails or even causes further complications, including cancer hyper-progression and immune-related adverse events. Along with countless translational studies of the PD-1 signaling pathway, there are currently close to 5,000 clinical trials for antibodies against PD-1 and its ligand, PD-L1, around 80% of which investigate combinations with other therapies. Nevertheless, more work is needed to better understand the PD-1 signaling pathway and to facilitate new and improved evidence-based combination strategies. In this work, we consolidate recent discoveries of PD-1 signaling mediators and their therapeutic potential in combination with anti-PD-1/PD-L1 agents. We focus on the phosphatases SHP2 and PTPN2; the kinases ITK, VRK2, GSK-3, and CDK4/6; and the signaling adaptor protein PAG. We discuss their biology both in cancer cells and T cells, with a focus on their role in relation to PD-1 to determine their potential in therapeutic combinations. The literature discussed here was obtained from a search of the published literature and ClinicalTrials.gov with the following key terms: checkpoint inhibition, cancer immunotherapy, PD-1, PD-L1, SHP2, PTPN2, ITK, VRK2, CDK4/6, GSK-3, and PAG. Together, we find that all of these proteins are logical and promising targets for combination therapy, and that with a deeper mechanistic understanding they have potential to improve the response rate and decrease adverse events when thoughtfully used in combination with checkpoint inhibitors.
Keywords: ITK; PD-1; PD-L1; SHP2; T cell.
Copyright © 2022 Moore, Strazza and Mor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.Oncologist. 2019 Feb;24(Suppl 1):S31-S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. Oncologist. 2019. PMID: 30819829 Free PMC article. Review.
-
Enhancing cancer immunotherapy: Exploring strategies to target the PD-1/PD-L1 axis and analyzing the associated patent, regulatory, and clinical trial landscape.Eur J Pharm Biopharm. 2024 Jul;200:114323. doi: 10.1016/j.ejpb.2024.114323. Epub 2024 May 15. Eur J Pharm Biopharm. 2024. PMID: 38754524 Review.
-
Generation, secretion and degradation of cancer immunotherapy target PD-L1.Cell Mol Life Sci. 2022 Jul 11;79(8):413. doi: 10.1007/s00018-022-04431-x. Cell Mol Life Sci. 2022. PMID: 35819633 Free PMC article. Review.
-
Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities.Front Immunol. 2020 Nov 5;11:598877. doi: 10.3389/fimmu.2020.598877. eCollection 2020. Front Immunol. 2020. PMID: 33250900 Free PMC article. Review.
Cited by
-
De Novo Purine Metabolism is a Metabolic Vulnerability of Cancers with Low p16 Expression.Cancer Res Commun. 2024 May 2;4(5):1174-1188. doi: 10.1158/2767-9764.CRC-23-0450. Cancer Res Commun. 2024. PMID: 38626341 Free PMC article.
-
Radiation drives tertiary lymphoid structures to reshape TME for synergized antitumour immunity.Expert Rev Mol Med. 2024 Oct 23;26:e30. doi: 10.1017/erm.2024.27. Expert Rev Mol Med. 2024. PMID: 39438247 Free PMC article. Review.
-
Exclusion of PD-1 from the immune synapse: A novel strategy to modulate T cell function.Mol Ther Oncol. 2024 Jun 17;32(3):200839. doi: 10.1016/j.omton.2024.200839. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39072290 Free PMC article.
-
Leukemia-intrinsic determinants of CAR-T response revealed by iterative in vivo genome-wide CRISPR screening.Nat Commun. 2023 Dec 5;14(1):8048. doi: 10.1038/s41467-023-43790-2. Nat Commun. 2023. PMID: 38052854 Free PMC article.
-
Cancer therapy by cyclin-dependent kinase inhibitors (CDKIs): bench to bedside.EXCLI J. 2024 Jun 4;23:862-882. doi: 10.17179/excli2024-7076. eCollection 2024. EXCLI J. 2024. PMID: 38983782 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

