Investigating the Mechanism of Germinal Center Shutdown
- PMID: 35911680
- PMCID: PMC9329532
- DOI: 10.3389/fimmu.2022.922318
Investigating the Mechanism of Germinal Center Shutdown
Abstract
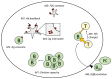
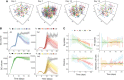
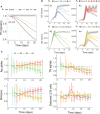
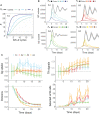
Germinal centers (GCs) are transient structures where affinity maturation of B cells gives rise to high affinity plasma and memory cells. The mechanism of GC shutdown is unclear, despite being an important phenomenon maintaining immune homeostasis. In this study, we used a mathematical model to identify mechanisms that can independently promote contraction of GCs leading to shutdown. We show that GC shutdown can be promoted by antigen consumption by B cells, antigen masking by soluble antibodies, alterations in follicular dendritic cell (FDC) network area, modulation of immune complex cycling rate constants, alterations in T follicular helper signaling, increased terminal differentiation and reduced B cell division capacity. Proposed mechanisms promoted GC contraction by ultimately decreasing the number of B cell divisions and recycling cells. Based on the in-silico predictions, we suggest a combination of experiments that can be potentially employed by future studies to unravel the mechanistic basis of GC shutdown such as measurements of the density of pMHC presentation of B cells, FDC network size per B cell, fraction of cells expressing differentiation markers. We also show that the identified mechanisms differentially affect the efficiency of GC reaction estimated based on the quantity and quality of resulting antibodies.
Keywords: antibody responses; chronic germinal centers; germinal center shutdown; mathematical modeling; vaccination.
Copyright © 2022 Arulraj, Binder and Meyer-Hermann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Antibody Mediated Intercommunication of Germinal Centers.Cells. 2022 Nov 19;11(22):3680. doi: 10.3390/cells11223680. Cells. 2022. PMID: 36429109 Free PMC article.
-
Germinal Centre Shutdown.Front Immunol. 2021 Jul 7;12:705240. doi: 10.3389/fimmu.2021.705240. eCollection 2021. Front Immunol. 2021. PMID: 34305944 Free PMC article. Review.
-
Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation.Immunity. 2010 Jul 23;33(1):84-95. doi: 10.1016/j.immuni.2010.07.005. Immunity. 2010. PMID: 20643339
-
Amount of antigen, T follicular helper cells and affinity of founder cells shape the diversity of germinal center B cells: A computational study.Front Immunol. 2023 Mar 13;14:1080853. doi: 10.3389/fimmu.2023.1080853. eCollection 2023. Front Immunol. 2023. PMID: 36993964 Free PMC article.
-
T cells and follicular dendritic cells in germinal center B-cell formation and selection.Immunol Rev. 2010 Sep;237(1):72-89. doi: 10.1111/j.1600-065X.2010.00937.x. Immunol Rev. 2010. PMID: 20727030 Review.
Cited by
-
Prolonging the delivery of influenza virus vaccine improves the quantity and quality of the induced immune responses in mice.Front Immunol. 2023 Oct 5;14:1249902. doi: 10.3389/fimmu.2023.1249902. eCollection 2023. Front Immunol. 2023. PMID: 37869002 Free PMC article.
-
Antibody Mediated Intercommunication of Germinal Centers.Cells. 2022 Nov 19;11(22):3680. doi: 10.3390/cells11223680. Cells. 2022. PMID: 36429109 Free PMC article.
-
A multiscale spatial modeling framework for the germinal center response.Front Immunol. 2024 May 30;15:1377303. doi: 10.3389/fimmu.2024.1377303. eCollection 2024. Front Immunol. 2024. PMID: 38881901 Free PMC article.
-
Immune landscape of tertiary lymphoid structures in hepatocellular carcinoma (HCC) treated with neoadjuvant immune checkpoint blockade.bioRxiv [Preprint]. 2023 Oct 26:2023.10.16.562104. doi: 10.1101/2023.10.16.562104. bioRxiv. 2023. PMID: 37904980 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

