Investigating the Role of the NLRP3 Inflammasome Pathway in Acute Intestinal Inflammation: Use of THP-1 Knockout Cell Lines in an Advanced Triple Culture Model
- PMID: 35911682
- PMCID: PMC9326178
- DOI: 10.3389/fimmu.2022.898039
Investigating the Role of the NLRP3 Inflammasome Pathway in Acute Intestinal Inflammation: Use of THP-1 Knockout Cell Lines in an Advanced Triple Culture Model
Abstract
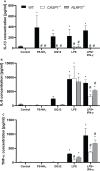
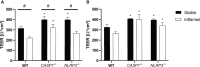
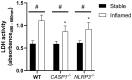
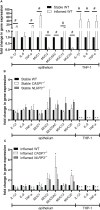
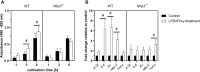
The NLRP3 inflammasome plays an important role in intestinal homeostasis as well as inflammation. However, in vivo studies investigating the role of the NLRP3 inflammasome in inflammatory bowel disease (IBD) report contrasting results, leaving it unclear if the NLRP3 inflammasome augments or attenuates intestinal inflammation. To investigate the role of the NLRP3/caspase-1 pathway in a model of acute intestinal inflammation, we modified a previously established in vitro triple culture model of the healthy and inflamed intestine (Caco-2/HT29-MTX-E12/THP-1). Using THP-1 knockout cell lines, we analyzed how the NLRP3 inflammasome and its downstream enzyme caspase-1 (CASP1) affect inflammatory parameters including barrier integrity and cytotoxicity, as well as gene expression and secretion of pro-inflammatory cytokines and mucus. Furthermore, we investigated differences in inflammation-mediated cytotoxicity towards enterocyte-like (Caco-2) or goblet-like (HT29-MTX-E12) epithelial cells. As a complementary approach, inflammation-related cytotoxicity and gene expression of cytokines was analyzed in intestinal tissue explants from wildtype (WT) and Nlrp3-/- mice. Induction of intestinal inflammation impaired the barrier, caused cytotoxicity, and altered gene expression of pro-inflammatory cytokines and mucins in vitro, while the knockout of NLRP3 and CASP1 in THP 1 cells led to attenuation of these inflammatory parameters. The knockout of CASP1 tended to show a slightly stronger attenuating effect compared to the NLRP3 knockout model. We also found that the inflammation-mediated death of goblet-like cells is NLRP3/caspase-1 dependent. Furthermore, inflammation-related cytotoxicity and upregulation of pro-inflammatory cytokines was present in ileal tissue explants from WT, but not Nlrp3-/- mice. The here presented observations indicate a pro-inflammatory and adverse role of the NLRP3 inflammasome in macrophages during acute intestinal inflammation.
Keywords: HT29-MTX-E12; IBD; barrier; caco-2; gastrointestinal tract; inflammatory disease; macrophages; mucus.
Copyright © 2022 Busch, Ramachandran, Wahle, Rossi and Schins.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Atox1 regulates macrophage polarization in intestinal inflammation via ROS-NLRP3 inflammasome pathway.J Transl Med. 2024 May 25;22(1):497. doi: 10.1186/s12967-024-05314-4. J Transl Med. 2024. PMID: 38796413 Free PMC article.
-
Calcium sensing receptor activation in THP-1 macrophages triggers NLRP3 inflammasome and human preadipose cell inflammation.Mol Cell Endocrinol. 2020 Feb 5;501:110654. doi: 10.1016/j.mce.2019.110654. Epub 2019 Nov 15. Mol Cell Endocrinol. 2020. PMID: 31734269
-
The Pathogenic Role of NLRP3 Inflammasome Activation in Inflammatory Bowel Diseases of Both Mice and Humans.J Crohns Colitis. 2017 Jun 1;11(6):737-750. doi: 10.1093/ecco-jcc/jjw219. J Crohns Colitis. 2017. PMID: 27993998 Free PMC article.
-
NLRP3: A Promising Therapeutic Target for Inflammatory Bowel Disease.Curr Drug Targets. 2023;24(14):1106-1116. doi: 10.2174/0113894501255960231101105113. Curr Drug Targets. 2023. PMID: 37946354 Review.
-
Polyphenols as NLRP3 inflammasome modulators in cardiometabolic diseases: a review of in vivo studies.Food Funct. 2023 Oct 30;14(21):9534-9553. doi: 10.1039/d3fo03015f. Food Funct. 2023. PMID: 37855750 Review.
Cited by
-
Development and assessment of an intestinal tri-cellular model to investigate the pro/anti-inflammatory potential of digested foods.Front Immunol. 2025 Feb 5;16:1545261. doi: 10.3389/fimmu.2025.1545261. eCollection 2025. Front Immunol. 2025. PMID: 39975553 Free PMC article.
-
Saharan dust induces NLRP3-dependent inflammatory cytokines in an alveolar air-liquid interface co-culture model.Part Fibre Toxicol. 2023 Oct 20;20(1):39. doi: 10.1186/s12989-023-00550-w. Part Fibre Toxicol. 2023. PMID: 37864207 Free PMC article.
-
Cross-talk between NLRP3 and AIM2 inflammasomes in macrophage activation by LPS and titanium ions.Mol Med. 2025 Jun 9;31(1):223. doi: 10.1186/s10020-025-01290-7. Mol Med. 2025. PMID: 40490723 Free PMC article.
-
Elevated risk of adverse effects from foodborne contaminants and drugs in inflammatory bowel disease: a review.Arch Toxicol. 2024 Nov;98(11):3519-3541. doi: 10.1007/s00204-024-03844-w. Epub 2024 Sep 9. Arch Toxicol. 2024. PMID: 39249550 Free PMC article. Review.
-
NLRP3 Inflammasome and Gut Dysbiosis Linking Diabetes Mellitus and Inflammatory Bowel Disease.Arch Intern Med Res. 2024;7(3):200-218. doi: 10.26502/aimr.0178. Epub 2024 Aug 31. Arch Intern Med Res. 2024. PMID: 39328924 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

