The Protective Effect of the Soluble Egg Antigen of Schistosoma japonicum in A Mouse Skin Transplantation Model
- PMID: 35911717
- PMCID: PMC9332893
- DOI: 10.3389/fimmu.2022.884006
The Protective Effect of the Soluble Egg Antigen of Schistosoma japonicum in A Mouse Skin Transplantation Model
Abstract
Background: Organ transplantation is currently an effective method for treating organ failure. Long-term use of immunosuppressive drugs has huge side effects, which severely restricts the long-term survival of patients. Schistosoma can affect the host's immune system by synthesizing, secreting, or excreting a variety of immunomodulatory molecules, but its role in transplantation was not well defined. In order to explore whether Schistosoma-related products can suppress rejection and induce long-term survival of the transplant, we used soluble egg antigen (SEA) of Schistosoma japonicum in mouse skin transplantation models.
Materials and methods: Each mouse was intraperitoneally injected with 100 μg of SEA three times a week for four consecutive weeks before allogenic skin transplant. Skin transplants were performed on day 0 to observe graft survival. Pathological examination of skin grafts was conducted 7 days post transplantation. The skin grafts were subjected to mRNA sequencing. Bioinformatics analysis was conducted and the expression of hub genes was verified by qPCR. Flow cytometry analysis was performed to evaluate the immune status and validate the results from bioinformatic analysis.
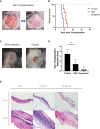
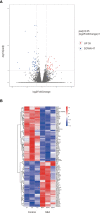
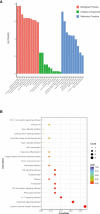
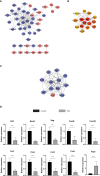
Results: The mean survival time (MST) of mouse skin grafts in the SEA-treated group was 11.67 ± 0.69 days, while that of the control group was 8.00 ± 0.36 days. Pathological analysis showed that Sj SEA treatment led to reduced inflammatory infiltration within skin grafts 7 days after allogenic skin transplantation. Bioinformatics analysis identified 86 DEGs between the Sj SEA treatment group and the control group, including 39 upregulated genes and 47 downregulated genes. Further analysis revealed that Sj SEA mediated regulation on cellular response to interferon-γ, activation of IL-17 signaling and chemokine signaling pathways, as well as cytokine-cytokine receptor interaction. Flow cytometry analysis showed that SEA treatment led to higher percentages of CD4+IL-4+ T cells and CD4+Foxp3+ T cells and decreased CD4+IFN-γ+ T cells in skin transplantation.
Conclusion: Sj SEA treatment suppressed rejection and prolonged skin graft survival by regulating immune responses. Sj SEA treatment might be a potential new therapeutic strategy to facilitate anti-rejection therapy and even to induce tolerance.
Keywords: Schistosoma japonicum; cytokine–cytokine receptor interaction; gene expression; skin graft; soluble egg antigen.
Copyright © 2022 Jiang, Li, Zhang, Zhou, Guo, Zhou, Ming.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Characterization of Schistosoma japonicum CP1412 protein as a novel member of the ribonuclease T2 molecule family with immune regulatory function.Parasit Vectors. 2017 Feb 17;10(1):89. doi: 10.1186/s13071-016-1962-y. Parasit Vectors. 2017. PMID: 28212670 Free PMC article.
-
Schistosoma japonicum Soluble Egg Antigen Protects Against Type 2 Diabetes in Lepr db/db Mice by Enhancing Regulatory T Cells and Th2 Cytokines.Front Immunol. 2019 Jun 26;10:1471. doi: 10.3389/fimmu.2019.01471. eCollection 2019. Front Immunol. 2019. PMID: 31297120 Free PMC article.
-
[Effects of soluble egg antigen and adult worm antigen of Schistosoma japonicum on differentiation of effector B cells of mice].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013 Oct;25(5):488-92. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013. PMID: 24490359 Chinese.
-
[Vaccination of mice with recombinant nucleic acid vaccine encoding the integral membrane protein Sj23 and cytokine IL-12 elicits specific immune responses against schistosoma japonica].Zhonghua Yi Xue Za Zhi. 2005 Jan 19;85(3):193-8. Zhonghua Yi Xue Za Zhi. 2005. PMID: 15854467 Chinese.
-
Characterization of a family of monoclonal antibodies which bind Schistosoma japonicum egg antigens and express an interstrain cross-reactive idiotype.Parasite Immunol. 1990 Mar;12(2):199-211. doi: 10.1111/j.1365-3024.1990.tb00948.x. Parasite Immunol. 1990. PMID: 1690872
Cited by
-
Schistosoma antigens: A future clinical magic bullet for autoimmune diseases?Parasite. 2024;31:68. doi: 10.1051/parasite/2024067. Epub 2024 Oct 31. Parasite. 2024. PMID: 39481080 Free PMC article. Review.
-
Helminth-derived molecules: Pathogenic and pharmacopeial roles.J Biomed Res. 2024 Sep 24;38(6):547-568. doi: 10.7555/JBR.38.20240177. J Biomed Res. 2024. PMID: 39314046 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

