The Therapeutic Potential of Targeting NIK in B Cell Malignancies
- PMID: 35911754
- PMCID: PMC9326486
- DOI: 10.3389/fimmu.2022.930986
The Therapeutic Potential of Targeting NIK in B Cell Malignancies
Abstract
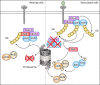
NF-κB-inducing kinase (NIK) is a key player in non-canonical NF-κB signaling, involved in several fundamental cellular processes, and is crucial for B cell function and development. In response to certain signals and ligands, such as CD40, BAFF and lymphotoxin-β activation, NIK protein stabilization and subsequent NF-κB activation is achieved. Overexpression or overactivation of NIK is associated with several malignancies, including activating mutations in multiple myeloma (MM) and gain-of-function in MALT lymphoma as a result of post-translational modifications. Consequently, drug discovery studies are devoted to pharmacologic modulation of NIK and development of specific novel small molecule inhibitors. However, disease-specific in vitro and in vivo studies investigating NIK inhibition are as of yet lacking, and clinical trials with NIK inhibitors remain to be initiated. In order to bridge the gap between bench and bedside, this review first briefly summarizes our current knowledge on NIK activation, functional activity and stability. Secondly, we compare current inhibitors targeting NIK based on efficacy and specificity, and provide a future perspective on the therapeutic potential of NIK inhibition in B cell malignancies.
Keywords: B cell malignancies; NIK; in vitro; in vivo; small molecule inhibitors; therapeutic targets.
Copyright © 2022 Haselager and Eldering.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

