ICAM-1 on Breast Cancer Cells Suppresses Lung Metastasis but Is Dispensable for Tumor Growth and Killing by Cytotoxic T Cells
- PMID: 35911772
- PMCID: PMC9328178
- DOI: 10.3389/fimmu.2022.849701
ICAM-1 on Breast Cancer Cells Suppresses Lung Metastasis but Is Dispensable for Tumor Growth and Killing by Cytotoxic T Cells
Abstract
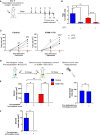
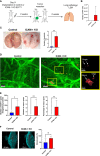
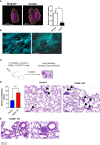
Breast tumors and their derived circulating cancer cells express the leukocyte β2 integrin ligand Intercellular adhesion molecule 1 (ICAM-1). We found that elevated ICAM-1 expression in breast cancer cells results in a favorable outcome and prolonged survival of breast cancer patients. We therefore assessed the direct in vivo contribution of ICAM-1 expressed by breast cancer cells to breast tumorigenesis and lung metastasis in syngeneic immunocompetent mice hosts using spontaneous and experimental models of the lung metastasis of the C57BL/6-derived E0771 cell line, a luminal B breast cancer subtype. Notably, the presence of ICAM-1 on E0771 did not alter tumor growth or the leukocyte composition in the tumor microenvironment. Interestingly, the elimination of Tregs led to the rapid killing of primary tumor cells independently of tumor ICAM-1 expression. The in vivo elimination of a primary E0771 tumor expressing the ovalbumin (OVA) model neoantigen by the OVA-specific OVA-tcr-I mice (OT-I) transgenic cytotoxic T lymphocytes (CTLs) also took place normally in the absence of ICAM-1 expression by E0771 breast cancer target cells. The whole lung imaging of these cells by light sheet microscopy (LSM) revealed that both Wild type (WT)- and ICAM-1-deficient E0771 cells were equally disseminated from resected tumors and accumulated inside the lung vasculature at similar magnitudes. ICAM-1-deficient breast cancer cells developed, however, much larger metastatic lesions than their control counterparts. Strikingly, the vast majority of these cells gave rise to intravascular tumor colonies both in spontaneous and experimental metastasis models. In the latter model, ICAM-1 expressing E0771- but not their ICAM-1-deficient counterparts were highly susceptible to elimination by neutrophils adoptively transferred from E0771 tumor-bearing donor mice. Ex vivo, neutrophils derived from tumor-bearing mice also killed cultured E0771 cells via ICAM-1-dependent interactions. Collectively, our results are a first indication that ICAM-1 expressed by metastatic breast cancer cells that expand inside the lung vasculature is involved in innate rather than in adaptive cancer cell killing. This is also a first indication that the breast tumor expression of ICAM-1 is not required for CTL-mediated killing but can function as a suppressor of intravascular breast cancer metastasis to lungs.
Keywords: adhesion; integrins; killing; metastasis; neutrophils; vasculature.
Copyright © 2022 Regev, Kizner, Roncato, Dadiani, Saini, Castro-Giner, Yajuk, Kozlovski, Levi, Addadi, Golani, Ben-Dor, Granot, Aceto and Alon.
Conflict of interest statement
NA is a paid consultant for companies with an interest in liquid biopsy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Adoptive transfer of natural killer cells promotes the anti-tumor efficacy of T cells.Clin Immunol. 2017 Apr;177:76-86. doi: 10.1016/j.clim.2016.06.013. Epub 2016 Jul 1. Clin Immunol. 2017. PMID: 27377534 Free PMC article.
-
Stimulation of beta1 integrin down-regulates ICAM-1 expression and ICAM-1-dependent adhesion of lung cancer cells through focal adhesion kinase.Cancer Res. 2001 Mar 1;61(5):2022-30. Cancer Res. 2001. PMID: 11280762
-
Steroid receptor coactivator-3 inhibition generates breast cancer antitumor immune microenvironment.Breast Cancer Res. 2022 Oct 31;24(1):73. doi: 10.1186/s13058-022-01568-2. Breast Cancer Res. 2022. PMID: 36316775 Free PMC article.
-
Lung ICAM-1 and ICAM-2 support spontaneous intravascular effector lymphocyte entrapment but are not required for neutrophil entrapment or emigration inside endotoxin-inflamed lungs.FASEB J. 2016 May;30(5):1767-78. doi: 10.1096/fj.201500046. Epub 2016 Jan 28. FASEB J. 2016. PMID: 26823454
-
Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control.J Immunol. 2006 Mar 1;176(5):2902-14. doi: 10.4049/jimmunol.176.5.2902. J Immunol. 2006. PMID: 16493048
Cited by
-
Integrating proteomics and network pharmacology to explore the relevant mechanism of Huangkui capsule in the treatment of chronic glomerulonephritis.Front Pharmacol. 2025 Apr 28;16:1560420. doi: 10.3389/fphar.2025.1560420. eCollection 2025. Front Pharmacol. 2025. PMID: 40356949 Free PMC article.
-
DCZ19931, a novel multi-targeting kinase inhibitor, inhibits ocular neovascularization.Sci Rep. 2022 Dec 13;12(1):21539. doi: 10.1038/s41598-022-25811-0. Sci Rep. 2022. PMID: 36513701 Free PMC article.
-
Teriparatide Does Not Exacerbate Bone Metastases in Breast Cancer Bone Metastasis Model.In Vivo. 2025 Jul-Aug;39(4):1932-1940. doi: 10.21873/invivo.13992. In Vivo. 2025. PMID: 40578986 Free PMC article.
-
Unveiling the intricate dynamics of the interplay between triple-negative breast cancer cells and the blood-brain barrier endothelium.Acta Neuropathol Commun. 2025 Aug 28;13(1):185. doi: 10.1186/s40478-025-01985-2. Acta Neuropathol Commun. 2025. PMID: 40877996 Free PMC article.
-
Role of ICAM1 in tumor immunity and prognosis of triple-negative breast cancer.Front Immunol. 2023 Aug 21;14:1176647. doi: 10.3389/fimmu.2023.1176647. eCollection 2023. Front Immunol. 2023. PMID: 37671167 Free PMC article.
References
-
- Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 Regulates Neutrophil Adhesion and Transcellular Migration of TNF-Alpha-Activated Vascular Endothelium Under Flow. Blood (2005) 106:584–92. doi: ICAM-1 Regulates Neutrophil Adhesion and Transcellular Migration of TNF-Alpha-Activated Vascular Endothelium Under Flow - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

