The Quest for Anti-α-Synuclein Antibody Specificity-Lessons Learnt From Flow Cytometry Analysis
- PMID: 35911883
- PMCID: PMC9334871
- DOI: 10.3389/fneur.2022.869103
The Quest for Anti-α-Synuclein Antibody Specificity-Lessons Learnt From Flow Cytometry Analysis
Abstract
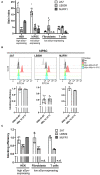
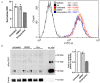
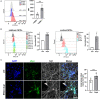
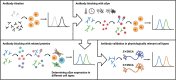
The accumulation of alpha-synuclein (aSyn) is the hallmark of a group of neurodegenerative conditions termed synucleopathies. Physiological functions of aSyn, including those outside of the CNS, remain elusive. However, a reliable and reproducible evaluation of aSyn protein expression in different cell types and especially in low-expressing cells is impeded by the existence of a huge variety of poorly characterized anti-aSyn antibodies and a lack of a routinely used sensitive detection methods. Here, we developed a robust flow cytometry-based workflow for aSyn detection and antibody validation. We test our workflow using three commercially available antibodies (MJFR1, LB509, and 2A7) in a variety of human cell types, including induced pluripotent stem cells, T lymphocytes, and fibroblasts, and provide a cell- and antibody-specific map for aSyn expression. Strikingly, we demonstrate a previously unobserved unspecificity of the LB509 antibody, while the MJFR1 clone revealed specific aSyn binding however with low sensitivity. On the other hand, we identified an aSyn-specific antibody clone 2A7 with an optimal sensitivity for detecting aSyn in a range of cell types, including those with low aSyn expression. We further utilize our workflow to demonstrate the ability of the 2A7 antibody to distinguish between physiological differences in aSyn expression in neuronal and non-neuronal cells from the cortical organoids, and in neural progenitors and midbrain dopaminergic neurons from healthy controls and in patients with Parkinson's disease who have aSyn gene locus duplication. Our results provide a proof of principle for the use of high-throughput flow cytometry-based analysis of aSyn and highlight the necessity of rigorous aSyn antibody validation to facilitate the research of aSyn physiology and pathology.
Keywords: T cell; alpha-synuclein; antibodies; antibody specificity; flow cytometry; synuclein antibodies; α-synuclein expression.
Copyright © 2022 Leupold, Sigutova, Gerasimova, Regensburger, Zundler, Zunke, Xiang, Winner and Prots.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Translocation of Distinct Alpha Synuclein Species from the Nucleus to Neuronal Processes during Neuronal Differentiation.Biomolecules. 2022 Aug 12;12(8):1108. doi: 10.3390/biom12081108. Biomolecules. 2022. PMID: 36009004 Free PMC article.
-
Interaction of Alpha Synuclein and Microtubule Organization Is Linked to Impaired Neuritic Integrity in Parkinson's Patient-Derived Neuronal Cells.Int J Mol Sci. 2022 Feb 5;23(3):1812. doi: 10.3390/ijms23031812. Int J Mol Sci. 2022. PMID: 35163733 Free PMC article.
-
Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain.J Virol. 2015 Dec 30;90(6):2767-82. doi: 10.1128/JVI.02949-15. J Virol. 2015. PMID: 26719256 Free PMC article.
-
Novel Immunotherapeutic Approaches to Target Alpha-Synuclein and Related Neuroinflammation in Parkinson's Disease.Cells. 2019 Jan 31;8(2):105. doi: 10.3390/cells8020105. Cells. 2019. PMID: 30708997 Free PMC article. Review.
-
⍺-Synuclein Structural Diversity and the Cellular Environment in ⍺-Synuclein Transmission Models and Humans.Neurotherapeutics. 2023 Jan;20(1):67-82. doi: 10.1007/s13311-023-01365-5. Epub 2023 Apr 13. Neurotherapeutics. 2023. PMID: 37052776 Free PMC article. Review.
Cited by
-
Protease-Sensitive and -Resistant Forms of Human and Murine Alpha-Synucleins in Distinct Brain Regions of Transgenic Mice (M83) Expressing the Human Mutated A53T Protein.Biomolecules. 2023 Dec 13;13(12):1788. doi: 10.3390/biom13121788. Biomolecules. 2023. PMID: 38136658 Free PMC article.
-
Immunization targeting diseased proteins in synucleinopathy and tauopathy: insights from clinical trials.Transl Neurodegener. 2025 Jul 1;14(1):33. doi: 10.1186/s40035-025-00490-9. Transl Neurodegener. 2025. PMID: 40588759 Free PMC article. Review.
-
Generation of iPSC Lines with Tagged α-Synuclein for Visualization of Endogenous Protein in Human Cellular Models of Neurodegenerative Disorders.eNeuro. 2025 Jun 23;12(6):ENEURO.0093-25.2025. doi: 10.1523/ENEURO.0093-25.2025. Print 2025 Jun. eNeuro. 2025. PMID: 40456614 Free PMC article.
-
Effect of hydrogen sulfide on alpha-synuclein aggregation and cell viability.Sci Rep. 2025 May 4;15(1):15597. doi: 10.1038/s41598-025-99794-z. Sci Rep. 2025. PMID: 40320462 Free PMC article.
References
-
- Kumar ST, Jagannath S, Francois C, Vanderstichele H, Stoops E, Lashuel HA. How specific are the conformation-specific α-synuclein antibodies? Characterization and validation of 16 α-synuclein conformation-specific antibodies using well-characterized preparations of α-synuclein monomers, fibrils and oligomers with distinct structures and morphology. Neurobiol Dis. (2020) 146:105086. 10.1016/j.nbd.2020.105086 - DOI - PubMed
LinkOut - more resources
Full Text Sources

