Non-small-cell lung cancer: how to manage RET-positive disease
- PMID: 35912003
- PMCID: PMC9281974
- DOI: 10.7573/dic.2022-1-5
Non-small-cell lung cancer: how to manage RET-positive disease
Abstract
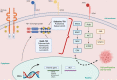
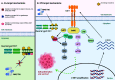
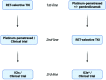
Targeted therapy has dramatically changed the history and outcomes of oncogene-addicted non-small-cell lung cancer (NSCLC). RET rearrangements are typically observed in about 1-2% of NSCLC, resulting in constitutive activation of downstream signalling pathways commonly involved in cell growth and survival. RET-positive NSCLCs are generally associated with young age, non-smoking history, a high rate of brain metastases at diagnosis and an immunologically 'cold' tumour microenvironment. Multi-kinase inhibitors, such as cabozantinib, lenvatinib and vandetanib, showed limited efficacy but significant toxicity mainly linked to off-target effects. In contrast, two RET-selective tyrosine kinase inhibitors (TKIs), selpercatinib and pralsetinib, demonstrated high response rates and manageable safety profiles, and have received FDA approval for the treatment of advanced RET-positive NSCLC regardless of previous lines of treatment. Despite the initial high response rate to RET-TKIs, most patients inevitably develop disease progression due to acquired resistance mechanisms by both on-target or off-target mechanisms. To date, new potent and selective next-generation RET-TKIs are currently being evaluated in ongoing clinical trials in order to overcome resistance and improve efficacy and blood-brain barrier crossing. Genomic recharacterization at progression could help guide treatment choice or enrolment in clinical trials of specific next-generation RET inhibitors. Here, we review the biology, clinicopathological characteristics, targeted therapies and mechanisms of resistance of advanced NSCLC harbouring RET fusions to provide treatment guidance for these patients.
Keywords: RET; next-generation sequencing; non-small-cell lung cancer; pralsetinib; selpercatinib; tyrosine kinase inhibitors.
Copyright © 2022 Andrini E, Mosca M, Galvani L, Sperandi F, Ricciuti B, Metro G, Lamberti G.
Conflict of interest statement
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/06/dic.2022-1-5-COI.pdf
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

