Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration
- PMID: 35912040
- PMCID: PMC9337215
- DOI: 10.3389/fragi.2022.926627
Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration
Abstract
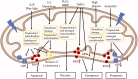
Retinal pigment epithelial (RPE) cells form a monolayer between the neuroretina and choroid. It has multiple important functions, including acting as outer blood-retina barrier, maintaining the function of neuroretina and photoreceptors, participating in the visual cycle and regulating retinal immune response. Due to high oxidative stress environment, RPE cells are vulnerable to dysfunction, cellular senescence, and cell death, which underlies RPE aging and age-related diseases, including age-related macular degeneration (AMD). Mitochondria are the powerhouse of cells and a major source of cellular reactive oxygen species (ROS) that contribute to mitochondrial DNA damage, cell death, senescence, and age-related diseases. Mitochondria also undergo dynamic changes including fission/fusion, biogenesis and mitophagy for quality control in response to stresses. The role of mitochondria, especially mitochondrial dynamics, in RPE aging and age-related diseases, is still unclear. In this review, we summarize the current understanding of mitochondrial function, biogenesis and especially dynamics such as morphological changes and mitophagy in RPE aging and age-related RPE diseases, as well as in the biological processes of RPE cellular senescence and cell death. We also discuss the current preclinical and clinical research efforts to prevent or treat RPE degeneration by restoring mitochondrial function and dynamics.
Keywords: RPE; age-related macula degeneration; aging; cell death; degeneration; mitochondria; senescense.
Copyright © 2022 Tong, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Mitochondrial quality control in non-exudative age-related macular degeneration: From molecular mechanisms to structural and functional recovery.Free Radic Biol Med. 2024 Jul;219:17-30. doi: 10.1016/j.freeradbiomed.2024.03.024. Epub 2024 Apr 4. Free Radic Biol Med. 2024. PMID: 38579938
-
Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration.Prog Retin Eye Res. 2020 Nov;79:100858. doi: 10.1016/j.preteyeres.2020.100858. Epub 2020 Apr 13. Prog Retin Eye Res. 2020. PMID: 32298788 Free PMC article. Review.
-
Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases.Cell Mol Neurobiol. 2023 Oct;43(7):3265-3276. doi: 10.1007/s10571-023-01383-z. Epub 2023 Jul 1. Cell Mol Neurobiol. 2023. PMID: 37391574 Free PMC article. Review.
-
Mitophagy initiates retrograde mitochondrial-nuclear signaling to guide retinal pigment cell heterogeneity.Autophagy. 2023 Mar;19(3):966-983. doi: 10.1080/15548627.2022.2109286. Epub 2022 Aug 13. Autophagy. 2023. PMID: 35921555 Free PMC article.
-
Mitochondria dynamics in the aged mice eye and the role in the RPE phagocytosis.Exp Eye Res. 2021 Dec;213:108800. doi: 10.1016/j.exer.2021.108800. Epub 2021 Oct 21. Exp Eye Res. 2021. PMID: 34688622
Cited by
-
Phloroglucinol Attenuates DNA Damage and Apoptosis Induced by Oxidative Stress in Human Retinal Pigment Epithelium ARPE-19 Cells by Blocking the Production of Mitochondrial ROS.Antioxidants (Basel). 2022 Nov 28;11(12):2353. doi: 10.3390/antiox11122353. Antioxidants (Basel). 2022. PMID: 36552561 Free PMC article.
-
Dysfunctional Autophagy, Proteostasis, and Mitochondria as a Prelude to Age-Related Macular Degeneration.Int J Mol Sci. 2023 May 15;24(10):8763. doi: 10.3390/ijms24108763. Int J Mol Sci. 2023. PMID: 37240109 Free PMC article. Review.
-
Potential Role of NUR77 in the Aging Retinal Pigment Epithelium and Age-Related Macular Degeneration.Adv Exp Med Biol. 2025;1468:165-169. doi: 10.1007/978-3-031-76550-6_27. Adv Exp Med Biol. 2025. PMID: 39930190 Review.
-
The Ercc1-/Δ mouse model of XFE progeroid syndrome undergoes accelerated retinal degeneration.Aging Cell. 2025 Mar;24(3):e14419. doi: 10.1111/acel.14419. Epub 2024 Nov 27. Aging Cell. 2025. PMID: 39604117 Free PMC article.
-
Metabolic states influence chicken retinal pigment epithelium cell fate decisions.Development. 2024 Aug 1;151(15):dev202462. doi: 10.1242/dev.202462. Epub 2024 Aug 9. Development. 2024. PMID: 39120084 Free PMC article.
References
-
- Ach T., Tolstik E., Messinger J. D., Zarubina A. V., Heintzmann R., Curcio C. A. (2015). Lipofuscin Redistribution and Loss Accompanied by Cytoskeletal Stress in Retinal Pigment Epithelium of Eyes with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 56, 3242–3252. 10.1167/iovs.14-16274 - DOI - PMC - PubMed
-
- Alaimo A., Liñares G. G., Bujjamer J. M., Gorojod R. M., Alcon S. P., Martínez J. H., et al. (2019). Toxicity of Blue Led Light and A2E Is Associated to Mitochondrial Dynamics Impairment in ARPE-19 Cells: Implications for Age-Related Macular Degeneration. Arch. Toxicol. 93, 1401–1415. 10.1007/s00204-019-02409-6 - DOI - PubMed
-
- An E., Lu X., Flippin J., Devaney J. M., Halligan B., Hoffman E., et al. (2007). Secreted Proteome Profiling in Human RPE Cell Cultures Derived from Donors with Age Related Macular Degeneration and Age Matched Healthy Donors J. Proteome Res. 2006, 5, 2599−2610. J. Proteome Res. 6, 1615. 10.1021/pr078003z - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

