Berberine protects cardiac cells against ferroptosis
- PMID: 35912047
- PMCID: PMC9333108
- DOI: 10.4103/tcmj.tcmj_236_21
Berberine protects cardiac cells against ferroptosis
Abstract
Objectives: Cardiovascular diseases are one of the primary causes of death. Cardiomyocyte loss is a significant feature of cardiac injury. Ferroptosis is iron-dependent cell death, which occurs due to excess iron and reactive oxygen species (ROS) accumulation causing lipid peroxidation, and subsequent cell death. Ferroptosis has been confirmed to mediate ischemia/reperfusion-induced cardiomyopathy and chemotherapy-induced cardiotoxicity. Berberine (BBR) has been proven to protect the heart from cardiomyopathies, including cardiac hypertrophy, heart failure, myocardial infarction, and arrhythmias. It protects cardiomyocytes from apoptosis and autophagy. However, the relation between BBR and ferroptosis is still unknown. This study aimed to confirm if BBR reduces cardiac cell loss via inhibiting ferroptosis.
Materials and methods: We used erastin and Ras-selective lethal small molecule 3 (RSL3) to establish a ferroptosis model in an H9c2 cardiomyoblast cell line and rat neonatal cardiomyocytes to prove that BBR has a protective effect on cardiac cells via inhibiting ferroptosis.
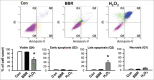
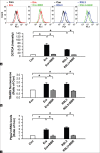
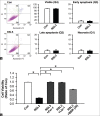
Results: In H9c2 cardiomyoblasts, the results showed that BBR reduced erastin and RSL3-induced cell viability loss. Moreover, BBR decreased ROS accumulation and lipid peroxidation in cells induced with ferroptosis. Furthermore, quantitative polymerase chain reaction results showed that Ptgs2 mRNA was reduced in BBR-treated cells. In rat neonatal cardiomyocytes, BBR reduced RSL3-induced loss of cell viability.
Conclusion: These results indicated that BBR inhibited ferroptosis via reducing ROS generation and reducing lipid peroxidation in erastin and RSL3-treated cardiac cells.
Keywords: Berberine; Cardiomyocyte; Ferroptosis; Ischemia-reperfusion injury; Lipid peroxidation.
Copyright: © 2022 Tzu Chi Medical Journal.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
