Connecting Metabolic Rewiring With Phenotype Switching in Melanoma
- PMID: 35912100
- PMCID: PMC9334657
- DOI: 10.3389/fcell.2022.930250
Connecting Metabolic Rewiring With Phenotype Switching in Melanoma
Abstract
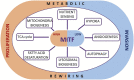
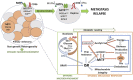
Melanoma is a complex and aggressive cancer type that contains different cell subpopulations displaying distinct phenotypes within the same tumor. Metabolic reprogramming, a hallmark of cell transformation, is essential for melanoma cells to adopt different phenotypic states necessary for adaptation to changes arising from a dynamic milieu and oncogenic mutations. Increasing evidence demonstrates how melanoma cells can exhibit distinct metabolic profiles depending on their specific phenotype, allowing adaptation to hostile microenvironmental conditions, such as hypoxia or nutrient depletion. For instance, increased glucose consumption and lipid anabolism are associated with proliferation, while a dependency on exogenous fatty acids and an oxidative state are linked to invasion and metastatic dissemination. How these different metabolic dependencies are integrated with specific cell phenotypes is poorly understood and little is known about metabolic changes underpinning melanoma metastasis. Recent evidence suggests that metabolic rewiring engaging transitions to invasion and metastatic progression may be dependent on several factors, such as specific oncogenic programs or lineage-restricted mechanisms controlling cell metabolism, intra-tumor microenvironmental cues and anatomical location of metastasis. In this review we highlight how the main molecular events supporting melanoma metabolic rewiring and phenotype-switching are parallel and interconnected events that dictate tumor progression and metastatic dissemination through interplay with the tumor microenvironment.
Keywords: MITF; drug resisitance; fatty acids; heterogeneity; melanoma; metabolic plasticity; mitochondria.
Copyright © 2022 Falletta, Goding and Vivas-García.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Lineage-Restricted Regulation of SCD and Fatty Acid Saturation by MITF Controls Melanoma Phenotypic Plasticity.Mol Cell. 2020 Jan 2;77(1):120-137.e9. doi: 10.1016/j.molcel.2019.10.014. Epub 2019 Nov 13. Mol Cell. 2020. PMID: 31733993 Free PMC article.
-
Metabolic buffering suppresses phenotype switching in cancer.bioRxiv [Preprint]. 2025 Apr 12:2025.04.07.647576. doi: 10.1101/2025.04.07.647576. bioRxiv. 2025. PMID: 40291724 Free PMC article. Preprint.
-
Understanding Molecular Mechanisms of Phenotype Switching and Crosstalk with TME to Reveal New Vulnerabilities of Melanoma.Cells. 2022 Mar 29;11(7):1157. doi: 10.3390/cells11071157. Cells. 2022. PMID: 35406721 Free PMC article. Review.
-
Lipid metabolic Reprogramming: Role in Melanoma Progression and Therapeutic Perspectives.Cancers (Basel). 2020 Oct 27;12(11):3147. doi: 10.3390/cancers12113147. Cancers (Basel). 2020. PMID: 33121001 Free PMC article. Review.
-
Microenvironment-derived factors driving metastatic plasticity in melanoma.Nat Commun. 2017 Feb 9;8:14343. doi: 10.1038/ncomms14343. Nat Commun. 2017. PMID: 28181494 Free PMC article.
Cited by
-
Alterations in Plasma Lipid Profiles Associated with Melanoma and Therapy Resistance.Int J Mol Sci. 2024 Jan 26;25(3):1558. doi: 10.3390/ijms25031558. Int J Mol Sci. 2024. PMID: 38338838 Free PMC article.
-
Differentiation status determines the effects of IFNγ on the expression of PD-L1 and immunomodulatory genes in melanoma.Cell Commun Signal. 2024 Dec 31;22(1):618. doi: 10.1186/s12964-024-01963-6. Cell Commun Signal. 2024. PMID: 39736644 Free PMC article.
-
Copper in melanoma: At the crossroad of protumorigenic and anticancer roles.Redox Biol. 2025 Apr;81:103552. doi: 10.1016/j.redox.2025.103552. Epub 2025 Feb 15. Redox Biol. 2025. PMID: 39970778 Free PMC article. Review.
-
Quantitative melanoma diagnosis using spectral phasor analysis of hyperspectral imaging from label-free slices.Front Oncol. 2023 Nov 17;13:1296826. doi: 10.3389/fonc.2023.1296826. eCollection 2023. Front Oncol. 2023. PMID: 38162497 Free PMC article.
-
BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers.Cancers (Basel). 2023 Aug 8;15(16):4026. doi: 10.3390/cancers15164026. Cancers (Basel). 2023. PMID: 37627054 Free PMC article. Review.
References
-
- Alicea G. M., Rebecca V. W., Goldman A. R., Fane M. E., Douglass S. M., Behera R., et al. (2020). Changes in Aged Fibroblast Lipid Metabolism Induce Age-dependent Melanoma Cell Resistance to Targeted Therapy via the Fatty Acid Transporter FATP2. Cancer Discov. 10, 1282–1285. 10.1158/2159-8290.CD-20-0329 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

