Chromatin and the Cellular Response to Particle Radiation-Induced Oxidative and Clustered DNA Damage
- PMID: 35912116
- PMCID: PMC9326100
- DOI: 10.3389/fcell.2022.910440
Chromatin and the Cellular Response to Particle Radiation-Induced Oxidative and Clustered DNA Damage
Abstract
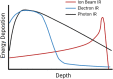
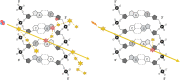
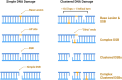
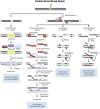
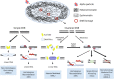
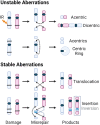
Exposure to environmental ionizing radiation is prevalent, with greatest lifetime doses typically from high Linear Energy Transfer (high-LET) alpha particles via the radioactive decay of radon gas in indoor air. Particle radiation is highly genotoxic, inducing DNA damage including oxidative base lesions and DNA double strand breaks. Due to the ionization density of high-LET radiation, the consequent damage is highly clustered wherein ≥2 distinct DNA lesions occur within 1-2 helical turns of one another. These multiply-damaged sites are difficult for eukaryotic cells to resolve either quickly or accurately, resulting in the persistence of DNA damage and/or the accumulation of mutations at a greater rate per absorbed dose, relative to lower LET radiation types. The proximity of the same and different types of DNA lesions to one another is challenging for DNA repair processes, with diverse pathways often confounding or interplaying with one another in complex ways. In this context, understanding the state of the higher order chromatin compaction and arrangements is essential, as it influences the density of damage produced by high-LET radiation and regulates the recruitment and activity of DNA repair factors. This review will summarize the latest research exploring the processes by which clustered DNA damage sites are induced, detected, and repaired in the context of chromatin.
Keywords: DNA repair; chromatin; clustered DNA damage; multiply-damaged sites; particle radiation.
Copyright © 2022 Danforth, Provencher and Goodarzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

