Efficacy and Safety of Vitamin D Adjuvant Therapy for Ulcerative Colitis: A Meta-Analysis
- PMID: 35912148
- PMCID: PMC9328974
- DOI: 10.1155/2022/6836942
Efficacy and Safety of Vitamin D Adjuvant Therapy for Ulcerative Colitis: A Meta-Analysis
Abstract
Objective: To examine the clinical efficacy and safety of Vitamin D in the treatment of ulcerative colitis in a systematic manner.
Methods: RCT studies on Vitamin D in the treatment of ulcerative colitis were searched from CNKI, Wanfang Data, PubMed, Cochrane Library, and Web of Science databases. RevMan 5.4 software was used for analysis.
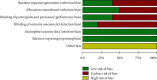
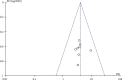
Results: 10 articles were included, including 1077 patients. Meta-analysis results showed that when clinical efficacy was used as the outcome index, the clinical efficacy of the oral vitamin group was higher than that of the conventional treatment group (OR = 4.07, 95% CI 2.64-6.27), and the difference was statistically significant (Z = 6.38, P < 0.00001). When the Mayo risk score was used as the outcome index, the difference was statistically significant, indicating that oral Vitamin D significantly reduced the Mayo risk score (MD: -0.41, CI = (-0.47, -0.34), Z = 13.09, P < 0.00001). Using the intestinal mucosal barrier as the outcome index, the results showed that (1) the MDA group (MD = -0.75, 95% CI (-0.96~-0.53), P < 0.00001), (2) the DAO group (MD = -1.17, 95% CI (-1.39-0.95), P < 0.00001), and the Vitamin D group could effectively improve intestinal mucosal barrier function after sensitivity analysis (MD = -1.00, 95% CI (-1.08-0.92), P < 0.00001). When inflammatory factors were used as outcome indicators, IL-6, TNF-α, and CRP groups had statistical significance (MD = -4.50, 95% CI (-5.13-3.87), P < 0.00001); MD = -7.27, 95% CI (18.96-5.58), P < 0.00001; and MD = -1.49, 95% CI (-1.76~-1.23), P < 0.00001, respectively). When the incidence of adverse reactions was used as the outcome indicator (OR = 0.73, 95% CI (0.34-1.32), P = 0.23), there was no significant difference between the two groups.
Conclusion: Vitamin D combined with mesalazine is effective in the treatment of ulcerative colitis, by improving the Mayo score and intestinal barrier function, and reducing inflammatory factors, with no significant safety difference. However, due to the quality of the included researches, more RCT researches needed to provide sufficient evidence to support clinical application. This study is registered with INPLASY 202250044.
Copyright © 2022 Xinyi Guo et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







Similar articles
-
Efficacy and safety of tanshinone IIA in combination with mesalazine in the treatment of ulcerative colitis: a Systematic review and meta-analysis.BMC Gastroenterol. 2024 Nov 15;24(1):410. doi: 10.1186/s12876-024-03496-1. BMC Gastroenterol. 2024. PMID: 39548391 Free PMC article.
-
[A preliminary study on the improved efficacy of mesalazine combined with vitamin D3 in ulcerative colitis].Zhonghua Nei Ke Za Zhi. 2022 Jul 1;61(7):785-792. doi: 10.3760/cma.j.cn112138-20210903-00613. Zhonghua Nei Ke Za Zhi. 2022. PMID: 35764562 Chinese.
-
The Efficacy and Safety of Mesalamine and Probiotics in Mild-to-Moderate Ulcerative Colitis: A Systematic Review and Meta-Analysis.Evid Based Complement Alternat Med. 2020 Mar 28;2020:6923609. doi: 10.1155/2020/6923609. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32308714 Free PMC article.
-
The correlation between serum 25-hydroxyvitamin D level and ulcerative colitis: a systematic review and meta-analysis.Eur J Gastroenterol Hepatol. 2023 Dec 1;35(12):1375-1381. doi: 10.1097/MEG.0000000000002670. Epub 2023 Oct 17. Eur J Gastroenterol Hepatol. 2023. PMID: 37851357
-
Factors associated with early outcomes following standardised therapy in children with ulcerative colitis (PROTECT): a multicentre inception cohort study.Lancet Gastroenterol Hepatol. 2017 Dec;2(12):855-868. doi: 10.1016/S2468-1253(17)30252-2. Epub 2017 Sep 20. Lancet Gastroenterol Hepatol. 2017. PMID: 28939374 Free PMC article.
Cited by
-
Nutrition, Nutritional Status, Micronutrients Deficiency, and Disease Course of Inflammatory Bowel Disease.Nutrients. 2023 Aug 31;15(17):3824. doi: 10.3390/nu15173824. Nutrients. 2023. PMID: 37686856 Free PMC article.
-
Nutrigenomic underpinnings of intestinal stem cells in inflammatory bowel disease and colorectal cancer development.Front Genet. 2024 Aug 30;15:1349717. doi: 10.3389/fgene.2024.1349717. eCollection 2024. Front Genet. 2024. PMID: 39280096 Free PMC article. Review.
-
Natural approaches for the management of ulcerative colitis: evidence of preclinical and clinical investigations.Nat Prod Bioprospect. 2024 Jul 30;14(1):42. doi: 10.1007/s13659-024-00463-x. Nat Prod Bioprospect. 2024. PMID: 39078427 Free PMC article. Review.
-
Management of paediatric ulcerative colitis, part 1: Ambulatory care-An updated evidence-based consensus guideline from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition and the European Crohn's and Colitis Organisation.J Pediatr Gastroenterol Nutr. 2025 Sep;81(3):765-815. doi: 10.1002/jpn3.70097. Epub 2025 Jul 18. J Pediatr Gastroenterol Nutr. 2025. PMID: 40677018 Free PMC article.
References
-
- Liang X., Yin F., Zhang X. Consensus opinion on diagnosis and treatment of inflammatory bowel disease (2018, Beijing) part interpretation of ulcerative colitis. Clinical Meta-Analysis . 2018;33(11):987–990.
-
- Adams S. M., Bornemann P. H. Ulcerative colitis. American Family Physician . 2013;87(10):699–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

