Clinical Efficacy of Retroauricular Injection of Methylprednisolone Sodium Succinate in the Treatment of Sudden Deafness with Type 2 Diabetes
- PMID: 35912152
- PMCID: PMC9337928
- DOI: 10.1155/2022/3097436
Clinical Efficacy of Retroauricular Injection of Methylprednisolone Sodium Succinate in the Treatment of Sudden Deafness with Type 2 Diabetes
Retraction in
-
Retracted: Clinical Efficacy of Retroauricular Injection of Methylprednisolone Sodium Succinate in the Treatment of Sudden Deafness with Type 2 Diabetes.Comput Math Methods Med. 2023 Sep 27;2023:9759346. doi: 10.1155/2023/9759346. eCollection 2023. Comput Math Methods Med. 2023. PMID: 37799432 Free PMC article.
Abstract
Background: The etiology of sudden deafness is still unclear. In recent years, people's life rhythm is getting faster and faster. Fatigue, environment, diet, psychology, and other factors have increased the morbidity rate of sudden deafness and improved the quality of life of patients. And work efficiency is greatly affected.
Aims: A study to investigate the clinical efficacy of postauricular injection of methylprednisolone sodium succinate in the treatment of sudden deafness with type 2 diabetes.
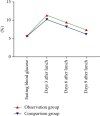
Materials and methods: Sixty patients with sudden deafness who were treated in our hospital from January 2018 to October 2020 were selected as the subjects of this prospective study and divided into 30 cases each in the comparison group and the observation group according to the random number remainder grouping method. The comparison group was treated conventionally, and the observation group was treated with postauricular injection of methylprednisolone sodium succinate on the basis of the comparison group. Patients in the two groups were observed and compared on the 3rd, 6th, and 9th days after treatment with pure-tone hearing threshold checks and regular monitoring of blood glucose, blood rheology, and other indexes.
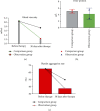
Results: On the 7th, 14th, and 30th days after treatment, the pure-tone audiometric thresholds of the two groups were gradually decreased, and the changes in the pure-tone audiometric thresholds in the observation group were greater than those in the control group. After lunch on the 6th day and after lunch on the 9th day, it was lower than that in the control group, and the difference was statistically significant (P < 0.05). 30 days after treatment, the blood viscosity, fibrin, and platelet aggregation rate of the observation group were significantly lower than those of the control group. After treatment, the clinical efficacy rate of the observation group was 96%, which was significantly higher than that of the control group, 80%, and the above differences were statistically significant (P < 0.05).
Conclusion: Treatment with postauricular injection of methylprednisolone sodium succinate has shown better therapeutic recovery in patients with sudden deafness, improved pure-tone hearing threshold, reduced risk of blood glucose elevation, and improved clinical outcomes for patients with sudden deafness, providing some reference for the treatment of patients with sudden deafness.
Copyright © 2022 Zhenbo Zhong et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
[Intratympanic dexamethasone vesus post-auricular subperiosteal injection of methylprednisolone treatment for sudden hearing loss].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017 Aug 20;31(16):1265-1268. doi: 10.13201/j.issn.1001-1781.2017.16.011. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017. PMID: 29798375 Clinical Trial. Chinese.
-
[Prospective randomised controlled observation of tympanic chamber injection of gangliosides in the treatment of refractory sudden deafness].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2025 Mar;39(3):218-222. doi: 10.13201/j.issn.2096-7993.2025.03.006. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2025. PMID: 40049636 Free PMC article. Clinical Trial. Chinese.
-
Postauricular injection of methylprednisolone sodium succinate as a salvage treatment for refractory sudden sensorineural hearing loss.Ir J Med Sci. 2021 Aug;190(3):1165-1172. doi: 10.1007/s11845-021-02610-6. Epub 2021 Apr 17. Ir J Med Sci. 2021. PMID: 33866519
-
Local vs Systemic Use of Steroids for Sudden Deafness with Diabetes: A Systematic Review and Meta-Analysis.Ear Nose Throat J. 2023 Apr 11:1455613231170090. doi: 10.1177/01455613231170090. Online ahead of print. Ear Nose Throat J. 2023. PMID: 37039340 Review.
-
Intratympanic steroids as primary initial treatment of idiopathic sudden sensorineural hearing loss. The Hospital Universitario Ramón y Cajal experience and review of the literature.Eur Arch Otorhinolaryngol. 2013 Nov;270(11):2823-32. doi: 10.1007/s00405-012-2306-y. Epub 2012 Dec 20. Eur Arch Otorhinolaryngol. 2013. PMID: 23254396 Review.
Cited by
-
Therapeutic efficacy of methylprednisolone sodium succinate via diverse administration routes for mid- to high-frequency sudden sensorineural hearing loss.World J Clin Cases. 2024 Jun 26;12(18):3321-3331. doi: 10.12998/wjcc.v12.i18.3321. World J Clin Cases. 2024. PMID: 38983415 Free PMC article.
-
Long-term efficacy of dexamethasone treatment via tympanic antrum catheterization for intractable Meniere's disease.Front Neurol. 2022 Dec 2;13:1056724. doi: 10.3389/fneur.2022.1056724. eCollection 2022. Front Neurol. 2022. PMID: 36530627 Free PMC article. Review.
-
Retracted: Clinical Efficacy of Retroauricular Injection of Methylprednisolone Sodium Succinate in the Treatment of Sudden Deafness with Type 2 Diabetes.Comput Math Methods Med. 2023 Sep 27;2023:9759346. doi: 10.1155/2023/9759346. eCollection 2023. Comput Math Methods Med. 2023. PMID: 37799432 Free PMC article.
References
-
- Herrera M., García Berrocal J. R., García Arumí A., Lavilla M. J., Plaza G., editors. Grupo de Trabajo de la Comisión de Audiología de la SEORL. Update on consensus on diagnosis and treatment of idiopathic sudden sensorineural hearing loss. Acta Otorrinolaringológica Española . 2019;70(5):290–300. doi: 10.1016/j.otorri.2018.04.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

