Highly Invasive Fluorescent/Bioluminescent Patient-Derived Orthotopic Model of Glioblastoma in Mice
- PMID: 35912166
- PMCID: PMC9326400
- DOI: 10.3389/fonc.2022.897839
Highly Invasive Fluorescent/Bioluminescent Patient-Derived Orthotopic Model of Glioblastoma in Mice
Erratum in
-
Corrigendum: Highly invasive fluorescent/bioluminescent patient-derived orthotopic model of glioblastoma in mice.Front Oncol. 2022 Sep 29;12:1040637. doi: 10.3389/fonc.2022.1040637. eCollection 2022. Front Oncol. 2022. PMID: 36248972 Free PMC article.
Abstract
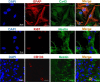
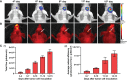
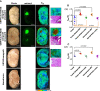
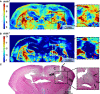
Development of the novel diagnostic and therapeutic approaches in neuro-oncology requires tumor models that closely reproduce the biological features of patients' tumors. Patient-derived xenografts (PDXs) are recognized as a valuable and the most "close-to-patient" tool for preclinical studies. However, their establishment is complicated by the factors related to both the surgical material and technique of the orthotopic implantation. The aim of this work was to develop a patient-derived glioblastoma multiform (GBM) model that stably co-expresses luciferase and a far-red fluorescent protein for monitoring of tumor progression in the brain and, using this model, to validate new diagnostic methods-macroscopic fluorescence lifetime imaging (macro-FLIM) and cross-polarization optical coherence tomography (CP OCT). The established model was similar to the original patient's GBM in terms of histological and immunohistochemical features and possessed reproducible growth in nude mice, which could be observed by both fluorescence and bioluminescence imaging. Our results demonstrated the high potential of macro-FLIM and CP OCT for intraoperative differentiation of GBM from the white matter. Thus, the dual-labeled PDX model of GBM proved to be an excellent approach for observation of tumor development by optical methods.
Keywords: FLIM (fluorescence lifetime imaging microscopy); fluorescence imaging; glioblastoma (GBM); patient-derived xenograft (PDX); primary cell line.
Copyright © 2022 Yuzhakova, Kiseleva, Shirmanova, Shcheslavskiy, Sachkova, Snopova, Bederina, Lukina, Dudenkova, Yusubalieva, Belovezhets, Matvienko and Baklaushev.
Conflict of interest statement
Author VS was employed by Becker&Hickl GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Golebiewska A, Hau A-C, Oudin A, Stieber D, Yabo YA, Baus V, et al. Patient-Derived Organoids and Orthotopic Xenografts of Primary and Recurrent Gliomas Represent Relevant Patient Avatars for Precision Oncology. Acta Neuropathol (2020) 140:919–49. doi: 10.1007/s00401-020-02226-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

