Targeting strategies in the treatment of fumarate hydratase deficient renal cell carcinoma
- PMID: 35912170
- PMCID: PMC9337267
- DOI: 10.3389/fonc.2022.906014
Targeting strategies in the treatment of fumarate hydratase deficient renal cell carcinoma
Abstract
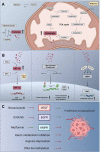
Fumarate hydratase (FH) - deficient renal cell carcinoma (FHdRCC) is a rare aggressive subtype of RCC caused by a germline or sporadic loss-of-function mutation in the FH gene. Here, we summarize how FH deficiency results in the accumulation of fumarate, which in turn leads to activation of hypoxia-inducible factor (HIF) through inhibition of prolyl hydroxylases. HIF promotes tumorigenesis by orchestrating a metabolic switch to glycolysis even under normoxia, a phenomenon well-known as the Warburg effect. HIF activates the transcription of many genes, including vascular endothelial growth factor (VEGF). Crosstalk between HIF and epidermal growth factor receptor (EGFR) has also been described as a tumor-promoting mechanism. In this review we discuss therapeutic options for FHdRCC with a focus on anti-angiogenesis and EGFR-blockade. We also address potential targets that arise within the metabolic escape routes taken by FH-deficient cells for cell growth and survival.
Keywords: bevacizumab; erlotinib; fumarate hydratase; fumarate hydratase deficient renal cell carcinoma; glucose; hereditary leiomyomatosis and renal cell cancer; metabolism; renal cell carcinoma (RCC).
Copyright © 2022 Lindner, Tulchiner, Seeber, Siska, Thurnher and Pichler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Fumarate hydratase deficient renal cell carcinoma and fumarate hydratase deficient-like renal cell carcinoma: Morphologic comparative study of 23 genetically tested cases.Cesk Patol. 2019 Fall;55(4):244-249. Cesk Patol. 2019. PMID: 31842557 English.
-
The oncometabolite fumarate promotes pseudohypoxia through noncanonical activation of NF-κB signaling.J Biol Chem. 2014 Aug 29;289(35):24691-9. doi: 10.1074/jbc.M114.568162. Epub 2014 Jul 15. J Biol Chem. 2014. PMID: 25028521 Free PMC article.
-
Fumarate Hydratase-deficient Renal Cell Carcinoma Is Strongly Correlated With Fumarate Hydratase Mutation and Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome.Am J Surg Pathol. 2016 Jul;40(7):865-75. doi: 10.1097/PAS.0000000000000617. Am J Surg Pathol. 2016. PMID: 26900816
-
Fumarate hydratase as a therapeutic target in renal cancer.Expert Opin Ther Targets. 2020 Sep;24(9):923-936. doi: 10.1080/14728222.2020.1804862. Epub 2020 Oct 6. Expert Opin Ther Targets. 2020. PMID: 32744123 Review.
-
[FH-deficient renal cell carcinoma expands the spectrum of renal papillary tumors].Pathologe. 2021 Nov;42(6):560-564. doi: 10.1007/s00292-021-00977-y. Epub 2021 Aug 27. Pathologe. 2021. PMID: 34448900 Free PMC article. Review. German.
Cited by
-
Fumarate hydratase (FH) and cancer: a paradigm of oncometabolism.Br J Cancer. 2023 Nov;129(10):1546-1557. doi: 10.1038/s41416-023-02412-w. Epub 2023 Sep 9. Br J Cancer. 2023. PMID: 37689804 Free PMC article. Review.
-
Synchronous Seminoma of Testis and Renal Cell Carcinoma: A Rare Case Report.Medicina (Kaunas). 2024 Sep 23;60(9):1553. doi: 10.3390/medicina60091553. Medicina (Kaunas). 2024. PMID: 39336594 Free PMC article.
-
Association between fumarate hydratase variant subtypes and the risk of HLRCC-associated renal cell carcinoma: systematic review and meta-analysis.Hum Genomics. 2025 Jul 21;19(1):83. doi: 10.1186/s40246-025-00797-8. Hum Genomics. 2025. PMID: 40691606 Free PMC article.
-
Comprehensive proteogenomic characterization of rare kidney tumors.Cell Rep Med. 2024 May 21;5(5):101547. doi: 10.1016/j.xcrm.2024.101547. Epub 2024 May 3. Cell Rep Med. 2024. PMID: 38703764 Free PMC article.
-
Altered metabolism in cancer: insights into energy pathways and therapeutic targets.Mol Cancer. 2024 Sep 18;23(1):203. doi: 10.1186/s12943-024-02119-3. Mol Cancer. 2024. PMID: 39294640 Free PMC article. Review.
References
-
- Leroy X, Zini L, Leteurtre E, Zerimech F, Porchet N, Aubert J-P, et al. . Morphologic subtyping of papillary renal cell carcinoma: correlation with prognosis and differential expression of MUC1 between the two subtypes. Mod Pathol (2002) 15(11):1126–30. doi: 10.1097/01.MP.0000036346.88874.25 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

