Evaluating the Therapeutic Potential of Idecabtagene Vicleucel in the Treatment of Multiple Myeloma: Evidence to Date
- PMID: 35912273
- PMCID: PMC9327779
- DOI: 10.2147/OTT.S305429
Evaluating the Therapeutic Potential of Idecabtagene Vicleucel in the Treatment of Multiple Myeloma: Evidence to Date
Abstract
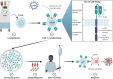
Over the past two decades, significant progress has been made in the diagnosis, risk assessment and treatment of patients with multiple myeloma, translating into remarkable improvements in survival outcomes. Yet, cure remains elusive, and almost all patients eventually experience relapse, particularly those with high-risk and refractory disease. Immune-based approaches have emerged as highly effective therapeutic options that have heralded a new era in the treatment of multiple myeloma. Idecabtagene vicleucel (ide-cel) is one such therapy that employs the use of genetically modified autologous T-cells to redirect immune activation in a tumor-directed fashion. It has yielded impressive responses even in patients with poor-risk disease and is the first chimeric antigen receptor (CAR) T-cell therapy to be approved for treatment in relapsed or refractory multiple myeloma. In this review, we examine the design and pharmacokinetics of ide-cel, audit evidence that led to its incorporation into the current treatment paradigm and provide insight into its clinical utilization with a focus on real-life intricacies.
Keywords: CAR-T; cytokine release syndrome; immunotherapy; multiple myeloma; neurotoxicity; resistance.
© 2022 Mann and Comenzo.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- American Society of Clinical Oncology. Multiple myeloma: statistics; 2021. Available from: https://www.cancer.net/cancer-types/multiple-myeloma/statistics. Accessed July 11, 2022.
-
- Perrot A, Lauwers-Cances V, Cazaubiel T, et al. Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: long-term follow-up analysis of the IFM 2009 trial. Blood. 2020;136(Supplement 1):39. doi:10.1182/blood-2020-134538 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous