Metamizole-Associated Risks in Decompensated Hepatic Cirrhosis
- PMID: 35912424
- PMCID: PMC9830680
- DOI: 10.3238/arztebl.m2022.0280
Metamizole-Associated Risks in Decompensated Hepatic Cirrhosis
Abstract
Background: Because of the increased risk of acute renal failure (ARF), the use of cyclooxygenase (COX) inhibitors is not recommended in patients with decompensated hepatic cirrhosis. Metamizole is not a classic COX inhibitor, but there are insufficient data to support its safe use. In this study, we investigate the effect of metamizole on the risk of ARF in these patients.
Methods: Metamizole use, ARF incidence, and patient mortality were examined in a large, retrospective, exploratory cohort and validated with data from a prospective registry.
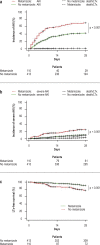
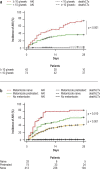
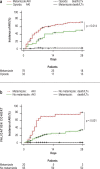
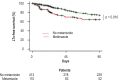
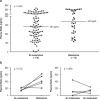
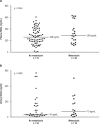
Results: 523 patients were evaluated in the exploratory cohort. Metamizole use at baseline was documented in 110 cases (21%) and was independently associated with the development of ARF, severe (grade 3) ARF, and lower survival without liver transplantation at follow-up on day 28 (HR: 2.2, p < 0.001; HR: 2.8, p < 0.001; and HR: 2.6, p < 0.001, respectively). Interestingly, the risk of ARF depended on the dose of metamizole administered (HR: 1.038, p < 0.001). Compared to patients who were treated with opioids, the rate of ARF was higher in the metamizole group (49% vs. 79%, p = 0.014). An increased risk of ARF with metamizole use was also demonstrated in the independent validation cohort (p < 0.001).
Conclusion: Metamizole therapy, especially at high doses, should only be used with a high level of caution in patients with decompensated cirrhosis.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

