The Clinical Use of Alzheimer's Disease Biomarkers in Patients with Mild Cognitive Impairment: A European Alzheimer's Disease Consortium Survey
- PMID: 35912743
- PMCID: PMC9535580
- DOI: 10.3233/JAD-220333
The Clinical Use of Alzheimer's Disease Biomarkers in Patients with Mild Cognitive Impairment: A European Alzheimer's Disease Consortium Survey
Abstract
Background: Recent advances occurred in the field of Alzheimer's disease (AD) biomarkers and the introduction of a research framework grounded on a biomarker-based definition of AD might have fostered an increased clinical use of AD biomarkers. For this reason, an up-to-date depiction of the clinical use of AD biomarkers is needed.
Objective: To investigate the clinical use of the main AD biomarkers in patients with mild cognitive impairment (MCI) by examining the beliefs and preferences of professionals (clinicians and biomarker experts) of the European Alzheimer's Disease Consortium (EADC).
Methods: 150 professionals filled in an online survey from May to September 2020. The investigated biomarkers were medial temporal lobe atrophy score (MTA) on structural MRI, typical AD (i.e., temporoparietal and posterior cingulate) hypometabolism on FDG-PET, CSF (Aβ42, p-tau, t-tau), amyloid-PET and tau-PET.
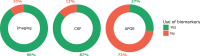
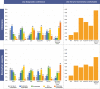
Results: The frequency of responders reporting a frequent-to-constant use of MTA (77%) is higher than that of those reporting a frequent-to-constant use of the other AD biomarkers (i.e.
, csf: 45%, p = 0.014; FDG-PET: 32%, p < 0.001; amyloid-PET: 8%, p < 0.001; and tau-PET: 2%, p < 0.001). CSF is considered the most valuable biomarker in terms of additional diagnostic value, followed by amyloid-PET, tau-PET, and typical AD hypometabolism on FDG-PET.
Conclusion: AD biomarkers are widely used across European memory clinics with a clinical research background for the diagnosis of MCI. Overall, we observed that CSF is currently considered as the most useful biomarker, followed by amyloid-PET.
Keywords: APOE; Alzheimer’s disease; FDG-PET; amyloid-PET; biomarkers; cerebrospinal fluid; clinical use; magnetic resonance imaging; mild cognitive impairment; tau-PET.
Conflict of interest statement
Authors’ disclosures available online (
Figures




References
-
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12, 292–323. - PMC - PubMed
-
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. - PMC - PubMed
-
- Ramusino MC, Garibotto V, Bacchin R, Altomare D, Dodich A, Assal F, Mendes A, Costa A, Tinazzi M, Morbelli SD, Bauckneht M, Picco A, Dottorini ME, Tranfaglia C, Farotti L, Salvadori N, Moretti D, Savelli G, Tarallo A, Nobili F, Parapini M, Cavaliere C, Salvatore E, Salvatore M, Boccardi M, Frisoni GB (2020) Incremental value of amyloid-PET versus CSF in the diagnosis of Alzheimer’s disease. Eur J Nucl Med Mol Imaging 47, 270–280. - PubMed
-
- Barthel H, Sabri O (2017) Clinical use and utility of amyloid imaging. J Nucl Med 58, 1711–1717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

