Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches
- PMID: 35912863
- PMCID: PMC9337829
- DOI: 10.1172/JCI161167
Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches
Abstract
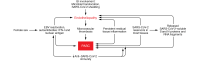
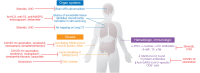
SARS-CoV-2-infected individuals may suffer a multi-organ system disorder known as "long COVID" or post-acute sequelae of SARS-CoV-2 infection (PASC). There are no standard treatments, the pathophysiology is unknown, and incidence varies by clinical phenotype. Acute COVID-19 correlates with biomarkers of systemic inflammation, hypercoagulability, and comorbidities that are less prominent in PASC. Macrovessel thrombosis, a hallmark of acute COVID-19, is less frequent in PASC. Female sex at birth is associated with reduced risk for acute COVID-19 progression, but with increased risk of PASC. Persistent microvascular endotheliopathy associated with cryptic SARS-CoV-2 tissue reservoirs has been implicated in PASC pathology. Autoantibodies, localized inflammation, and reactivation of latent pathogens may also be involved, potentially leading to microvascular thrombosis, as documented in multiple PASC tissues. Diagnostic assays illuminating possible therapeutic targets are discussed.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

