Rosa hybrida Petal Extract Exhibits Antitumor Effects by Abrogating Tumor Progression and Angiogenesis in Bladder Cancer Both In Vivo and In Vitro
- PMID: 35912937
- PMCID: PMC9421223
- DOI: 10.1177/15347354221114337
Rosa hybrida Petal Extract Exhibits Antitumor Effects by Abrogating Tumor Progression and Angiogenesis in Bladder Cancer Both In Vivo and In Vitro
Abstract
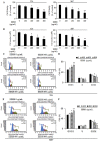
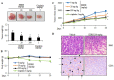
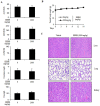
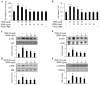
The edible Rosa hybrida (RH) petal is utilized in functional foods and cosmetics. Although the biological function of RH petal extract is known, mechanism of action studies involving tumor-associated angiogenesis have not yet been reported. Herein, we investigated the regulatory effect of the ethanol extract of RH petal (EERH) on tumor growth and tumor angiogenesis against bladder cancer. EERH treatment inhibited the bladder carcinoma T24 cell and 5637 cell proliferation because of G1-phase cell cycle arrest by inducing p21WAF1 expression and reducing cyclins/CDKs level. EERH regulated signaling pathways differently in both cells. EERH-stimulated suppression of T24 and 5637 cell migration and invasion was associated with the decline in transcription factor-mediated MMP-9 expression. EERH oral administration to xenograft mice reduced tumor growth. Furthermore, no obvious toxicity was observed in acute toxicity test. Decreased CD31 levels in EERH-treated tumor tissues led to examine the angiogenic response. EERH alleviated VEGF-stimulated tube formation and proliferation by downregulating the VEGFR2/eNOS/AKT/ERK1/2 cascade in HUVECs. EERH impeded migration and invasion of VEGF-induced HUVECs, which is attributed to the repressed MMP-2 expression. Suppression of neo-microvessel sprouting, induced by VEGF, was verified by treatment with EERH using the ex vivo aortic ring assay. Finally, kaempferol was identified as the main active compound of EERH. The present study demonstrated that EERH may aid the development of antitumor agents against bladder cancer.
Keywords: bladder cancer; bladder cancer cells; ethanol extract of Rosa hybrida; kaempferol; tumor angiogenesis; xenograft mice.
Conflict of interest statement
Figures





References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. - PubMed
-
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388:2796-2810. - PubMed
-
- Smith AB, Deal AM, Woods ME, et al. Muscle-invasive bladder cancer: evaluating treatment and survival in the National Cancer Data Base. BJU Int. 2014;114:719-726. - PubMed
-
- Schneider AK, Chevalier MF, Derré L. The multifaceted immune regulation of bladder cancer. Nat Rev Urol. 2019;16:613-630. - PubMed
-
- Black PC, Dinney CP. Bladder cancer angiogenesis and metastasis–translation from murine model to clinical trial. Cancer Metastasis Rev. 2007;26:623-634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

