Time-Dependent Biosensor Fluorescence as a Measure of Bacterial Arsenic Uptake Kinetics and Its Inhibition by Dissolved Organic Matter
- PMID: 35913152
- PMCID: PMC9397108
- DOI: 10.1128/aem.00891-22
Time-Dependent Biosensor Fluorescence as a Measure of Bacterial Arsenic Uptake Kinetics and Its Inhibition by Dissolved Organic Matter
Abstract
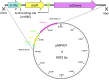
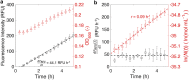
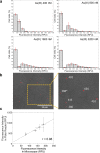
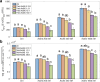
Microbe-mediated transformations of arsenic (As) often require As to be taken up into cells prior to enzymatic reaction. Despite the importance of these microbial reactions for As speciation and toxicity, understanding of how As bioavailability and uptake are regulated by aspects of extracellular water chemistry, notably dissolved organic matter (DOM), remains limited. Whole-cell biosensors utilizing fluorescent proteins are increasingly used for high-throughput quantification of the bioavailable fraction of As in water. Here, we present a mathematical framework for interpreting the time series of biosensor fluorescence as a measure of As uptake kinetics, which we used to evaluate the effects of different forms of DOM on uptake of trivalent arsenite. We found that thiol-containing organic compounds significantly inhibited uptake of arsenite into cells, possibly through the formation of aqueous complexes between arsenite and thiol ligands. While there was no evidence for competitive interactions between arsenite and low-molecular-weight neutral molecules (urea, glycine, and glyceraldehyde) for uptake through the aquaglyceroporin channel GlpF, which mediates transport of arsenite across cell membranes, there was evidence that labile DOM fractions may inhibit arsenite uptake through a catabolite repression-like mechanism. The observation of significant inhibition of arsenite uptake at DOM/As ratios commonly encountered in wetland pore waters suggests that DOM may be an important control on the microbial uptake of arsenite in the environment, with aspects of DOM quality playing an important role in the extent of inhibition. IMPORTANCE The speciation and toxicity of arsenic in environments like rice paddy soils and groundwater aquifers are controlled by microbe-mediated reactions. These reactions often require As to be taken up into cells prior to enzymatic reaction, but there is limited understanding of how microbial arsenic uptake is affected by variations in water chemistry. In this study, we explored the effect of dissolved organic matter (DOM) quantity and quality on microbial As uptake, with a focus on the role of thiol functional groups that are well known to form aqueous complexes with arsenic. We developed a quantitative framework for interpreting fluorescence time series from whole-cell biosensors and used this technique to evaluate effects of DOM on the rates of microbial arsenic uptake. We show that thiol-containing compounds significantly decrease rates of As uptake into microbial cells at environmentally relevant DOM/As ratios, revealing the importance of DOM quality in regulating arsenic uptake, and subsequent biotransformation, in the environment.
Keywords: arsenic; arsenite; bioavailability; biosensors; complexation; dissolved organic matter; kinetics; microbial uptake; modeling; thiols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effects of organic sulfur and arsenite/dissolved organic matter ratios on arsenite complexation with dissolved organic matter.Chemosphere. 2022 Sep;302:134770. doi: 10.1016/j.chemosphere.2022.134770. Epub 2022 Apr 29. Chemosphere. 2022. PMID: 35500636
-
Repression of microbial arsenite uptake and methylation by dissolved organic carbon.Environ Sci Technol Lett. 2024 Aug 13;11(8):838-844. doi: 10.1021/acs.estlett.4c00400. Epub 2024 Jul 15. Environ Sci Technol Lett. 2024. PMID: 40881500 Free PMC article.
-
Effects of different dissolved organic matter on microbial communities and arsenic mobilization in aquifers.J Hazard Mater. 2021 Jun 5;411:125146. doi: 10.1016/j.jhazmat.2021.125146. Epub 2021 Jan 14. J Hazard Mater. 2021. PMID: 33485230
-
Review on the interactions of arsenic, iron (oxy)(hydr)oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system.Chemosphere. 2022 Jan;286(Pt 2):131790. doi: 10.1016/j.chemosphere.2021.131790. Epub 2021 Aug 5. Chemosphere. 2022. PMID: 34388870 Review.
-
The ecology of arsenic.Science. 2003 May 9;300(5621):939-44. doi: 10.1126/science.1081903. Science. 2003. PMID: 12738852 Review.
References
-
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Kline J, Siddique AB, Shahriar H, Uddin MN, van Geen A, Mey JL, Balac O, Graziano JH. 2016. Child intelligence and reductions in water arsenic and manganese: a two-year follow-up study in Bangladesh. Environ Health Perspect 124:1114–1120. 10.1289/ehp.1509974. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

