LPS O Antigen Plays a Key Role in Klebsiella pneumoniae Capsule Retention
- PMID: 35913154
- PMCID: PMC9431683
- DOI: 10.1128/spectrum.01517-21
LPS O Antigen Plays a Key Role in Klebsiella pneumoniae Capsule Retention
Abstract
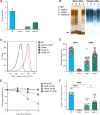
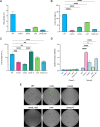
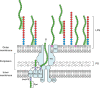
Despite the importance of encapsulation in bacterial pathogenesis, the biochemical mechanisms and forces that underpin retention of capsule by encapsulated bacteria are poorly understood. In Gram-negative bacteria, there may be interactions between lipopolysaccharide (LPS) core and capsule polymers, between capsule polymers with retained acyl carriers and the outer membrane, and in some bacteria, between the capsule polymers and Wzi, an outer membrane protein lectin. Our transposon studies in Klebsiella pneumoniae B5055 identified additional genes that, when insertionally inactivated, resulted in reduced encapsulation. Inactivation of the gene waaL, which encodes the ligase responsible for attaching the repeated O antigen of LPS to the LPS core, resulted in a significant reduction in capsule retention, measured by atomic force microscopy. This reduction in encapsulation was associated with increased sensitivity to human serum and decreased virulence in a murine model of respiratory infection and, paradoxically, with increased biofilm formation. The capsule in the WaaL mutant was physically smaller than that of the Wzi mutant of K. pneumoniae B5055. These results suggest that interactions between surface carbohydrate polymers may enhance encapsulation, a key phenotype in bacterial virulence, and provide another target for the development of antimicrobials that may avoid resistance issues associated with growth inhibition. IMPORTANCE Bacterial capsules, typically comprised of complex sugars, enable pathogens to avoid key host responses to infection, including phagocytosis. These capsules are synthesized within the bacteria, exported through the outer envelope, and then secured to the external surface of the organism by a force or forces that are incompletely described. This study shows that in the important hospital pathogen Klebsiella pneumoniae, the polysaccharide capsule is retained by interactions with other surface sugars, especially the repeated sugar molecule of the LPS molecule in Gram-negative bacteria known as "O antigen." This O antigen is joined to the LPS molecule by ligation, and loss of the enzyme responsible for ligation, a protein called WaaL, results in reduced encapsulation. Since capsules are essential to the virulence of many pathogens, WaaL might provide a target for new antimicrobial development, critical to the control of pathogens like K. pneumoniae that have become highly drug resistant.
Keywords: Klebsiella; LPS; O antigen; capsule; encapsulation; retention; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The ionic interaction of Klebsiella pneumoniae K2 capsule and core lipopolysaccharide.Microbiology (Reading). 2006 Jun;152(Pt 6):1807-1818. doi: 10.1099/mic.0.28611-0. Microbiology (Reading). 2006. PMID: 16735743
-
Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes.PLoS One. 2013;8(2):e56847. doi: 10.1371/journal.pone.0056847. Epub 2013 Feb 15. PLoS One. 2013. PMID: 23457627 Free PMC article.
-
A second galacturonic acid transferase is required for core lipopolysaccharide biosynthesis and complete capsule association with the cell surface in Klebsiella pneumoniae.J Bacteriol. 2007 Feb;189(3):1128-37. doi: 10.1128/JB.01489-06. Epub 2006 Dec 1. J Bacteriol. 2007. PMID: 17142396 Free PMC article.
-
Klebsiella pneumoniae: Going on the Offense with a Strong Defense.Microbiol Mol Biol Rev. 2016 Jun 15;80(3):629-61. doi: 10.1128/MMBR.00078-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27307579 Free PMC article. Review.
-
Molecular pathogenesis of Klebsiella pneumoniae.Future Microbiol. 2014;9(9):1071-81. doi: 10.2217/fmb.14.48. Future Microbiol. 2014. PMID: 25340836 Review.
Cited by
-
Transposon mutagenesis screen in Klebsiella pneumoniae identifies genetic determinants required for growth in human urine and serum.Elife. 2024 Aug 27;12:RP88971. doi: 10.7554/eLife.88971. Elife. 2024. PMID: 39189918 Free PMC article.
-
Klebsiella pneumoniae evolution in the gut leads to spontaneous capsule loss and decreased virulence potential.mBio. 2025 May 14;16(5):e0236224. doi: 10.1128/mbio.02362-24. Epub 2025 Mar 31. mBio. 2025. PMID: 40162782 Free PMC article.
-
Probiotic Characterization of Lactiplantibacillus paraplantarum SDN1.2 and Its Anti-Inflammatory Effect on Klebsiella pneumoniae-Infected Mammary Glands.Vet Sci. 2025 Apr 1;12(4):323. doi: 10.3390/vetsci12040323. Vet Sci. 2025. PMID: 40284825 Free PMC article.
-
Synergy between Group 2 capsules and lipopolysaccharide underpins serum resistance in extra-intestinal pathogenic Escherichia coli.Microbiology (Reading). 2024 Aug;170(8):001493. doi: 10.1099/mic.0.001493. Microbiology (Reading). 2024. PMID: 39177453 Free PMC article.
-
Adaptive evolution of carbapenem-resistant hypervirulent Klebsiella pneumoniae in the urinary tract of a single patient.Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2400446121. doi: 10.1073/pnas.2400446121. Epub 2024 Aug 16. Proc Natl Acad Sci U S A. 2024. PMID: 39150777 Free PMC article.
References
-
- Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. - DOI - PMC - PubMed
-
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi: 10.1016/S1473-3099(10)70143-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

