Plant-Microbe Interaction: Mining the Impact of Native Bacillus amyloliquefaciens WS-10 on Tobacco Bacterial Wilt Disease and Rhizosphere Microbial Communities
- PMID: 35913211
- PMCID: PMC9430121
- DOI: 10.1128/spectrum.01471-22
Plant-Microbe Interaction: Mining the Impact of Native Bacillus amyloliquefaciens WS-10 on Tobacco Bacterial Wilt Disease and Rhizosphere Microbial Communities
Abstract
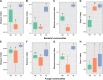
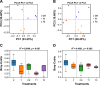
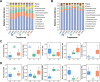
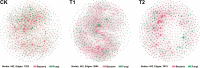
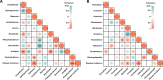
Ralstonia solanacearum, the causative agent of bacterial wilt disease, has been a major threat to tobacco production globally. Several control methods have failed. Thus, it is imperative to find effective management for this disease. The biocontrol agent Bacillus amyloliquefaciens WS-10 displayed a significant control effect due to biofilm formation, and secretion of hydrolytic enzymes and exopolysaccharides. In addition, strain WS-10 can produce antimicrobial compounds, which was confirmed by the presence of genes encoding antimicrobial lipopeptides (fengycin, iturin, surfactin, and bacillomycinD) and polyketides (difficidin, bacilysin, bacillibactin, and bacillaene). Strain WS-10 successfully colonized tobacco plant roots and rhizosphere soil and suppressed the incidence of bacterial wilt disease up to 72.02% by reducing the R. solanacearum population dynamic in rhizosphere soil. Plant-microbe interaction was considered a key driver of disease outcome. To further explore the impact of strain WS-10 on rhizosphere microbial communities, V3-V4 and ITS1 variable regions of 16S and ITS rRNA were amplified, respectively. Results revealed that strain WS-10 influences the rhizosphere microbial communities and dramatically changed the diversity and composition of rhizosphere microbial communities. Interestingly, the relative abundance of genus Ralstonia significantly decreased when treated with strain WS-10. A complex microbial co-occurrence network was present in a diseased state, and the introduction of strain WS-10 significantly changed the structure of rhizosphere microbiota. This study suggests that strain WS-10 can be used as a novel biocontrol agent to attain sustainability in disease management due to its intense antibacterial activity, efficient colonization in the host plant, and ability to transform the microbial community structure toward a healthy state. IMPORTANCE The plant rhizosphere acts as the first line of defense against the invasion of pathogens. The perturbation in the rhizosphere microbiome is directly related to plant health and disease development. The introduction of beneficial microorganisms in the soil shifted the rhizosphere microbiome, induced resistance in plants, and suppressed the incidence of soilborne disease. Bacillus sp. is widely used as a biocontrol agent against soilborne diseases due to its ability to produce broad-spectrum antimicrobial compounds and colonization with the host plant. In our study, we found that the application of native Bacillus amyloliquefaciens WS-10 significantly suppressed the incidence of tobacco bacterial wilt disease by shifting the rhizosphere microbiome and reducing the interaction between rhizosphere microorganisms and bacterial wilt pathogen.
Keywords: Ralstonia solanacearum; biological control; disease incidence; microbiome; plant pathogens; plant-microbe interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
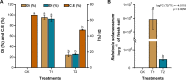


Similar articles
-
Ralstonia solanacearum infection induces tobacco root to secrete chemoattractants to recruit antagonistic bacteria and defensive compounds to inhibit pathogen.Pest Manag Sci. 2025 Apr;81(4):1817-1828. doi: 10.1002/ps.8581. Epub 2024 Dec 13. Pest Manag Sci. 2025. PMID: 39673161
-
Microbial Cross-Talk: Dissecting the Core Microbiota Associated With Flue-Cured Tobacco (Nicotiana tabacum) Plants Under Healthy and Diseased State.Front Microbiol. 2022 Apr 14;13:845310. doi: 10.3389/fmicb.2022.845310. eCollection 2022. Front Microbiol. 2022. PMID: 35495684 Free PMC article.
-
The Endophytic Root Microbiome Is Different in Healthy and Ralstonia solanacearum-Infected Plants and Is Regulated by a Consortium Containing Beneficial Endophytic Bacteria.Microbiol Spectr. 2023 Feb 14;11(1):e0203122. doi: 10.1128/spectrum.02031-22. Epub 2022 Dec 14. Microbiol Spectr. 2023. PMID: 36515552 Free PMC article.
-
Harvesting the complex pathways of antibiotic production and resistance of soil bacilli for optimizing plant microbiome.FEMS Microbiol Ecol. 2020 Sep 1;96(9):fiaa142. doi: 10.1093/femsec/fiaa142. FEMS Microbiol Ecol. 2020. PMID: 32672816 Review.
-
Plant and soil-associated microbiome dynamics determine the fate of bacterial wilt pathogen Ralstonia solanacearum.Planta. 2023 Aug 1;258(3):57. doi: 10.1007/s00425-023-04209-w. Planta. 2023. PMID: 37524889 Review.
Cited by
-
Ralstonia solanacearum Infection Drives the Assembly and Functional Adaptation of Potato Rhizosphere Microbial Communities.Plant Pathol J. 2024 Oct;40(5):498-511. doi: 10.5423/PPJ.OA.06.2024.0086. Epub 2024 Oct 1. Plant Pathol J. 2024. PMID: 39397304 Free PMC article.
-
Exploring the Relationship Between Biochar Pore Structure and Microbial Community Composition in Promoting Tobacco Growth.Plants (Basel). 2024 Oct 22;13(21):2952. doi: 10.3390/plants13212952. Plants (Basel). 2024. PMID: 39519871 Free PMC article.
-
Bacillus amyloliquefaciens LM-1 Affects Multiple Cell Biological Processes in Magnaporthe oryzae to Suppress Rice Blast.Microorganisms. 2024 Jun 20;12(6):1246. doi: 10.3390/microorganisms12061246. Microorganisms. 2024. PMID: 38930628 Free PMC article.
-
Fungal gut microbiota dysbiosis in systemic lupus erythematosus.Front Microbiol. 2023 Apr 5;14:1149311. doi: 10.3389/fmicb.2023.1149311. eCollection 2023. Front Microbiol. 2023. PMID: 37089568 Free PMC article.
-
Different Response Mechanisms of Rhizosphere Microbial Communities in Two Species of Amorphophallus to Pectobacterium carotovorum subsp. carotovorum Infection.Plant Pathol J. 2023 Apr;39(2):207-219. doi: 10.5423/PPJ.OA.12.2022.0157. Epub 2023 Apr 1. Plant Pathol J. 2023. PMID: 37019830 Free PMC article.
References
-
- Li C, Ahmed W, Li D, Yu L, Xu L, Xu T, Zhao Z. 2022. Biochar suppresses bacterial wilt disease of flue-cured tobacco by improving soil health and functional diversity of rhizosphere microorganisms. Appl Soil Ecol 171:104314. doi:10.1016/j.apsoil.2021.104314. - DOI
-
- Wu X, Li H, Wang Y, Zhang X. 2020. Effects of bio-organic fertiliser fortified by Bacillus cereus QJ-1 on tobacco bacterial wilt control and soil quality improvement. Biocontrol Science and Technology 30:351–369. doi:10.1080/09583157.2020.1711870. - DOI
-
- Li Y, Ren K, Zou C, Xie J, He X, Chen Y, Hu B, Shen J, Hu X, Chen J, Xia Z, Wu Y, Jin Y. 2019. Effects of ferrous iron toxicity on agronomic, physiological, and quality indices of flue‐cured tobacco. Agronomy J 111:2193–2206. doi:10.2134/agronj2018.12.0786. - DOI
-
- Cai Q, Zhou G, Ahmed W, Cao Y, Zhao M, Li Z, Zhao Z. 2021. Study on the relationship between bacterial wilt and rhizospheric microbial diversity of flue-cured tobacco cultivars. Eur J Plant Pathol 160:265–276. doi:10.1007/s10658-021-02237-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

