Conformational Changes in Herpes Simplex Virus Glycoprotein C
- PMID: 35913218
- PMCID: PMC9400475
- DOI: 10.1128/jvi.00163-22
Conformational Changes in Herpes Simplex Virus Glycoprotein C
Abstract
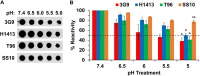
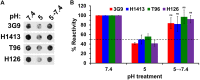
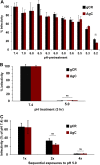
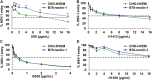
Low endosomal pH facilitates herpesvirus entry in a cell-specific manner. Herpes simplex virus 1 (HSV-1) causes significant morbidity and death in humans worldwide. HSV-1 enters cells by low-pH and neutral-pH pathways. Low-pH-induced conformational changes in the HSV envelope glycoprotein B (gB) may mediate membrane fusion during viral entry. HSV-1 gC, a 511-amino acid, type I integral membrane glycoprotein, mediates HSV-1 attachment to host cell surface glycosaminoglycans, but this interaction is not essential for viral entry. We previously demonstrated that gC regulates low-pH viral entry independent of its known role in cell attachment. Low-pH-triggered conformational changes in gB occur at a lower pH when gC is absent, suggesting that gC positively regulates gB conformational changes. Here, we demonstrate that mildly acidic pH triggers conformational changes in gC itself. Low-pH treatment of virions induced antigenic changes in distinct gC epitopes, and those changes were reversible. One of these gC epitopes is recognized by a monoclonal antibody that binds to a linear sequence that includes residues within gC amino acids 33 to 123. This antibody inhibited low-pH entry of HSV, suggesting that its gC N-terminal epitope is particularly important. We propose that gC plays a critical role in HSV entry through a low-pH endocytosis pathway, which is a major entry route in human epithelial cells. IMPORTANCE Herpesviruses are ubiquitous pathogens that cause lifelong latent infections and are characterized by multiple entry pathways. The HSV envelope gC regulates HSV entry by a low-pH entry route. The fusion protein gB undergoes pH-triggered conformational changes that are facilitated by gC. Here, we report that gC itself undergoes a conformational change at low pH. A monoclonal antibody to gC that binds to a region that undergoes pH-induced changes also selectively inhibits HSV low-pH entry, corroborating the importance of gC in the low-pH entry pathway. This study illustrates the complex role of endosomal pH during HSV entry and provides novel insights into the functions of gC.
Keywords: conformational change; endocytosis; endosomal pH; gC; glycoproteins; herpes simplex virus; herpesviruses; membrane fusion; viral entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Herpes Simplex Virus Glycoprotein C Regulates Low-pH Entry.mSphere. 2020 Feb 5;5(1):e00826-19. doi: 10.1128/mSphere.00826-19. mSphere. 2020. PMID: 32024702 Free PMC article.
-
Mildly Acidic pH Triggers an Irreversible Conformational Change in the Fusion Domain of Herpes Simplex Virus 1 Glycoprotein B and Inactivation of Viral Entry.J Virol. 2017 Feb 14;91(5):e02123-16. doi: 10.1128/JVI.02123-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28003487 Free PMC article.
-
Acidic pH Mediates Changes in Antigenic and Oligomeric Conformation of Herpes Simplex Virus gB and Is a Determinant of Cell-Specific Entry.J Virol. 2018 Aug 16;92(17):e01034-18. doi: 10.1128/JVI.01034-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925660 Free PMC article.
-
Herpesvirus Entry into Host Cells Mediated by Endosomal Low pH.Traffic. 2016 Sep;17(9):965-75. doi: 10.1111/tra.12408. Epub 2016 May 24. Traffic. 2016. PMID: 27126894 Free PMC article. Review.
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
Cited by
-
Anti-Herpetic Activity of Killer Peptide (KP): An In Vitro Study.Int J Mol Sci. 2024 Oct 1;25(19):10602. doi: 10.3390/ijms251910602. Int J Mol Sci. 2024. PMID: 39408931 Free PMC article.
-
Herpes simplex virus 1 envelope glycoprotein C shields glycoprotein D to protect virions from entry-blocking antibodies.J Virol. 2025 Apr 15;99(4):e0009025. doi: 10.1128/jvi.00090-25. Epub 2025 Mar 26. J Virol. 2025. PMID: 40135897 Free PMC article.
-
Prunella vulgaris polysaccharide inhibits herpes simplex virus infection by blocking TLR-mediated NF-κB activation.Chin Med. 2024 Jan 8;19(1):6. doi: 10.1186/s13020-023-00865-y. Chin Med. 2024. PMID: 38185640 Free PMC article.
-
Herpes Simplex Virus 1 Glycoprotein B from a Hyperfusogenic Virus Mediates Enhanced Cell-Cell Fusion.Viruses. 2024 Feb 4;16(2):251. doi: 10.3390/v16020251. Viruses. 2024. PMID: 38400027 Free PMC article.
-
Multiple Sites on Glycoprotein H (gH) Functionally Interact with the gB Fusion Protein to Promote Fusion during Herpes Simplex Virus (HSV) Entry.mBio. 2023 Feb 28;14(1):e0336822. doi: 10.1128/mbio.03368-22. Epub 2023 Jan 11. mBio. 2023. PMID: 36629412 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

