Immunogenicity and safety of SARS-CoV-2 mRNA vaccine in patients with nephrotic syndrome receiving immunosuppressive agents
- PMID: 35913562
- PMCID: PMC9340689
- DOI: 10.1007/s00467-022-05633-y
Immunogenicity and safety of SARS-CoV-2 mRNA vaccine in patients with nephrotic syndrome receiving immunosuppressive agents
Abstract
Background: As there are no large-scale reports of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) mRNA vaccination in patients with nephrotic syndrome using immunosuppressive agents, we conducted the prospective study.
Methods: SARS-CoV-2 mRNA vaccines were administered to patients with nephrotic syndrome receiving immunosuppressive agents. The titers of SARS-CoV-2 spike protein receptor-binding domain antibodies were measured before and after vaccination. We evaluated factors associated with antibody titers after vaccination and analyzed adverse events.
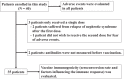
Results: We enrolled 40 patients and evaluated vaccine immunogenicity in 35 of them. Seroconversion (> 0.8 U/mL) was achieved in all patients, and the median antibody titer was 598 U/mL (interquartile range, 89-1380 U/mL). Patients using mycophenolate mofetil (MMF) showed lower antibody titers than those who were not (median: 272 U/mL vs. 2660 U/mL, p = 0.0002), and serum immunoglobulin G (IgG) levels showed a weak linear relationship with antibody titers (R2 = 0.16). No breakthrough infections were noted. Three patients (7.5%) suffered from a relapse of nephrotic syndrome (2 and 3 days, respectively, after the first dose and 8 days after the second dose), two of whom had a history of relapse within 6 months before the vaccination.
Conclusions: The SARS-CoV-2 mRNA vaccine was immunogenic in patients with nephrotic syndrome using immunosuppressive agents, although the use of MMF and low levels of serum IgG were associated with lower antibody titers after vaccination. Patients with high disease activity may experience a relapse of nephrotic syndrome after vaccination. A higher resolution version of the Graphical abstract is available as Supplementary information.
Keywords: Mycophenolate mofetil; Nephrotic syndrome; Relapse; SARS-CoV-2 S antibody; Seroconversion; Serum IgG.
© 2022. The Author(s), under exclusive licence to International Pediatric Nephrology Association.
Conflict of interest statement
Koichi Kamei has received research funding from the Terumo Foundation for Life Sciences and Arts and the Public Foundation of Vaccination Research Center; donations from Chugai Pharmaceutical Co. Ltd., Astellas Pharma Inc., Ono Pharmaceutical Co. Ltd, Teijin Pharma Ltd., Shionogi & Co. Ltd., and Otsuka Pharmaceutical Co. Ltd.; and lecture fees from Tanabe Mitsubishi Pharma Corp., Baxter Ltd., and Zenyaku Kogyo Co., Ltd. All other authors have no potential conflicts of interest to disclose. The first draft of the manuscript was written by Koichi Kamei.
Figures


Similar articles
-
Immunogenicity and safety of SARS-CoV-2 vaccine with immunosuppressive agents.Pediatr Int. 2022 Jan;64(1):e15331. doi: 10.1111/ped.15331. Pediatr Int. 2022. PMID: 36331234 Free PMC article.
-
Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients.J Hepatol. 2022 Jul;77(1):152-162. doi: 10.1016/j.jhep.2022.02.015. Epub 2022 Mar 10. J Hepatol. 2022. PMID: 35283215 Free PMC article.
-
Ongoing Mycophenolate Treatment Impairs Anti-SARS-CoV-2 Vaccination Response in Patients Affected by Chronic Inflammatory Autoimmune Diseases or Liver Transplantation Recipients: Results of the RIVALSA Prospective Cohort.Viruses. 2022 Aug 12;14(8):1766. doi: 10.3390/v14081766. Viruses. 2022. PMID: 36016388 Free PMC article.
-
Immunogenicity and Safety of SARS-CoV-2 Vaccination in Patients With Rheumatic Diseases: A Systematic Review and Meta-analysis.J Clin Rheumatol. 2022 Dec 1;28(8):381-389. doi: 10.1097/RHU.0000000000001871. Epub 2022 Jun 8. J Clin Rheumatol. 2022. PMID: 35660717
-
SARS-CoV-2 vaccination in patients with inflammatory bowel disease: A systemic review and meta-analysis.J Chin Med Assoc. 2022 Apr 1;85(4):421-430. doi: 10.1097/JCMA.0000000000000682. J Chin Med Assoc. 2022. PMID: 34974509
Cited by
-
Seroprevalence of SARS-CoV-2 antibodies in children with nephrotic syndrome and chronic kidney disease: a cross-sectional study from India.Pediatr Nephrol. 2025 Feb;40(2):441-447. doi: 10.1007/s00467-024-06534-y. Epub 2024 Sep 27. Pediatr Nephrol. 2025. PMID: 39327265
-
IPNA clinical practice recommendations on care of pediatric patients with pre-existing kidney disease during seasonal outbreak of COVID-19.Pediatr Nephrol. 2025 May;40(5):1795-1815. doi: 10.1007/s00467-024-06565-5. Epub 2024 Dec 29. Pediatr Nephrol. 2025. PMID: 39733391 Free PMC article. Review.
References
-
- Raja MA, Mendoza MA, Villavicencio A, Anjan S, Reynolds JM, Kittipibul V, Fernandez A, Guerra G, Camargo JF, Simkins J, Morris MI, Abbo LA, Natori Y. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando) 2021;35:100588. doi: 10.1016/j.trre.2020.100588. - DOI - PMC - PubMed
-
- Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L'Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M, ptbnet COVID-19 Study Group COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. - DOI - PMC - PubMed
-
- Viner RM, Mytton OT, Bonell C, Melendez-Torres GJ, Ward J, Hudson L, Waddington C, Thomas J, Russell S, van der Klis F, Koirala A, Ladhani S, Panovska-Griffiths J, Davies NG, Booy R, Eggo RM. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

