SILCS-RNA: Toward a Structure-Based Drug Design Approach for Targeting RNAs with Small Molecules
- PMID: 35913731
- PMCID: PMC9474704
- DOI: 10.1021/acs.jctc.2c00381
SILCS-RNA: Toward a Structure-Based Drug Design Approach for Targeting RNAs with Small Molecules
Abstract
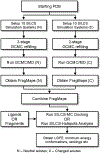
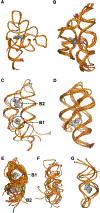
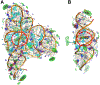
RNA molecules can act as potential drug targets in different diseases, as their dysregulated expression or misfolding can alter various cellular processes. Noncoding RNAs account for ∼70% of the human genome, and these molecules can have complex tertiary structures that present a great opportunity for targeting by small molecules. In the present study, the site identification by ligand competitive saturation (SILCS) computational approach is extended to target RNA, termed SILCS-RNA. Extensions to the method include an enhanced oscillating excess chemical potential protocol for the grand canonical Monte Carlo calculations and individual simulations of the neutral and charged solutes from which the SILCS functional group affinity maps (FragMaps) are calculated for subsequent binding site identification and docking calculations. The method is developed and evaluated against seven RNA targets and their reported small molecule ligands. SILCS-RNA provides a detailed characterization of the functional group affinity pattern in the small molecule binding sites, recapitulating the types of functional groups present in the ligands. The developed method is also shown to be useful for identification of new potential binding sites and identifying ligand moieties that contribute to binding, granular information that can facilitate ligand design. However, limitations in the method are evident including the ability to map the regions of binding sites occupied by ligand phosphate moieties and to fully account for the wide range of conformational heterogeneity in RNA associated with binding of different small molecules, emphasizing inherent challenges associated with applying computer-aided drug design methods to RNA. While limitations are present, the current study indicates how the SILCS-RNA approach may enhance drug discovery efforts targeting RNAs with small molecules.
Conflict of interest statement
Conflict of Interest
ADM Jr. is co-founder and CSO of SilcsBio LLC.
Figures

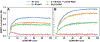






Similar articles
-
Reproducing crystal binding modes of ligand functional groups using Site-Identification by Ligand Competitive Saturation (SILCS) simulations.J Chem Inf Model. 2011 Apr 25;51(4):877-96. doi: 10.1021/ci100462t. Epub 2011 Apr 1. J Chem Inf Model. 2011. PMID: 21456594 Free PMC article.
-
Identification and characterization of fragment binding sites for allosteric ligand design using the site identification by ligand competitive saturation hotspots approach (SILCS-Hotspots).Biochim Biophys Acta Gen Subj. 2020 Apr;1864(4):129519. doi: 10.1016/j.bbagen.2020.129519. Epub 2020 Jan 3. Biochim Biophys Acta Gen Subj. 2020. PMID: 31911242 Free PMC article.
-
Inclusion of multiple fragment types in the site identification by ligand competitive saturation (SILCS) approach.J Chem Inf Model. 2013 Dec 23;53(12):3384-98. doi: 10.1021/ci4005628. Epub 2013 Nov 25. J Chem Inf Model. 2013. PMID: 24245913 Free PMC article.
-
Frameworks for targeting RNA with small molecules.J Biol Chem. 2021 Jan-Jun;296:100191. doi: 10.1074/jbc.REV120.015203. Epub 2020 Dec 20. J Biol Chem. 2021. PMID: 33334887 Free PMC article. Review.
-
Characterization of protein-ligand interaction sites using experimental and computational methods.Curr Opin Drug Discov Devel. 2006 May;9(3):354-62. Curr Opin Drug Discov Devel. 2006. PMID: 16729732 Review.
Cited by
-
Combined Physics- and Machine-Learning-Based Method to Identify Druggable Binding Sites Using SILCS-Hotspots.J Chem Inf Model. 2024 Oct 14;64(19):7743-7757. doi: 10.1021/acs.jcim.4c01189. Epub 2024 Sep 16. J Chem Inf Model. 2024. PMID: 39283165
-
GPU-specific algorithms for improved solute sampling in grand canonical Monte Carlo simulations.J Comput Chem. 2023 Jul 30;44(20):1719-1732. doi: 10.1002/jcc.27121. Epub 2023 Apr 24. J Comput Chem. 2023. PMID: 37093676 Free PMC article.
-
Efficient Sampling of Cavity Hydration in Proteins with Nonequilibrium Grand Canonical Monte Carlo and Polarizable Force Fields.J Chem Theory Comput. 2024 Mar 12;20(5):1897-1911. doi: 10.1021/acs.jctc.4c00013. Epub 2024 Feb 28. J Chem Theory Comput. 2024. PMID: 38417108 Free PMC article.
-
Identifying small-molecules binding sites in RNA conformational ensembles with SHAMAN.Nat Commun. 2024 Jul 8;15(1):5725. doi: 10.1038/s41467-024-49638-7. Nat Commun. 2024. PMID: 38977675 Free PMC article.
-
Enhancing SILCS-MC via GPU Acceleration and Ligand Conformational Optimization with Genetic and Parallel Tempering Algorithms.J Phys Chem B. 2024 Aug 1;128(30):7362-7375. doi: 10.1021/acs.jpcb.4c03045. Epub 2024 Jul 20. J Phys Chem B. 2024. PMID: 39031121 Free PMC article.
References
-
- Macalino SJ; Gosu V; Hong S; Choi S, Role of computer-aided drug design in modern drug discovery. Arch Pharm Res 2015, 38 (9), 1686–701. - PubMed
-
- Mullard A, Small molecules against RNA targets attract big backers. Nat Rev Drug Discov 2017, 16 (12), 813–815. - PubMed
-
- Petrone J; DeFrancesco L, Small molecules get the message. Nat Biotechnol 2018, 36 (9), 787–790. - PubMed
-
- Crooke ST; Witztum JL; Bennett CF; Baker BF, RNA-Targeted Therapeutics. Cell Metab 2018, 27 (4), 714–739. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
