Validity and reliability of the Patient-Reported Outcomes Measurement Information System (PROMIS®) using computerized adaptive testing in patients with advanced chronic kidney disease
- PMID: 35913734
- PMCID: PMC10157750
- DOI: 10.1093/ndt/gfac231
Validity and reliability of the Patient-Reported Outcomes Measurement Information System (PROMIS®) using computerized adaptive testing in patients with advanced chronic kidney disease
Abstract
Background: The Patient-Reported Outcomes Measurement Information System (PROMIS®) has been recommended for computerized adaptive testing (CAT) of health-related quality of life. This study compared the content, validity, and reliability of seven PROMIS CATs to the 12-item Short-Form Health Survey (SF-12) in patients with advanced chronic kidney disease.
Methods: Adult patients with chronic kidney disease and an estimated glomerular filtration rate under 30 mL/min/1.73 m2 who were not receiving dialysis treatment completed seven PROMIS CATs (assessing physical function, pain interference, fatigue, sleep disturbance, anxiety, depression, and the ability to participate in social roles and activities), the SF-12, and the PROMIS Pain Intensity single item and Dialysis Symptom Index at inclusion and 2 weeks. A content comparison was performed between PROMIS CATs and the SF-12. Construct validity of PROMIS CATs was assessed using Pearson's correlations. We assessed the test-retest reliability of all patient-reported outcome measures by calculating the intraclass correlation coefficient and minimal detectable change.
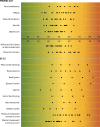
Results: In total, 207 patients participated in the study. A median of 45 items (10 minutes) were completed for PROMIS CATs. All PROMIS CATs showed evidence of sufficient construct validity. PROMIS CATs, most SF-12 domains and summary scores, and Dialysis Symptom Index showed sufficient test-retest reliability (intraclass correlation coefficient ≥ 0.70). PROMIS CATs had a lower minimal detectable change compared with the SF-12 (range, 5.7-7.4 compared with 11.3-21.7 across domains, respectively).
Conclusion: PROMIS CATs showed sufficient construct validity and test-retest reliability in patients with advanced chronic kidney disease. PROMIS CATs required more items but showed better reliability than the SF-12. Future research is needed to investigate the feasibility of PROMIS CATs for routine nephrology care.
Keywords: chronic kidney disease (CKD); minimal detectable change; patient-reported outcome measures (PROMs); reliability; validity.
© The Author(s) 2022. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
There are no financial or other conflicts of interest to declare. The results presented in this article have not been published previously in whole or part, except in abstract format.
Figures


Similar articles
-
Responsiveness and minimal important change of seven PROMIS computerized adaptive tests (CAT) in patients with advanced chronic kidney disease.J Patient Rep Outcomes. 2023 Apr 4;7(1):35. doi: 10.1186/s41687-023-00574-y. J Patient Rep Outcomes. 2023. PMID: 37016107 Free PMC article.
-
Validation of the PROMIS sleep disturbance item bank computer adaptive test (CAT) in patients on renal replacement therapy.Sleep Med. 2022 Feb;90:36-43. doi: 10.1016/j.sleep.2022.01.001. Epub 2022 Jan 10. Sleep Med. 2022. PMID: 35091171
-
Which type of PROMs to use in MS routine clinical care: The validity of PROMIS CAT questionnaires.Mult Scler Relat Disord. 2025 Mar;95:106320. doi: 10.1016/j.msard.2025.106320. Epub 2025 Feb 4. Mult Scler Relat Disord. 2025. PMID: 39933276
-
Recommendations for Patient-Reported Outcomes Measurement Information System pediatric measures in youth with chronic pain: a COnsensus-based Standards for the selection of health Measurement INstruments systematic review of measurement properties.Pain. 2024 Feb 1;165(2):258-295. doi: 10.1097/j.pain.0000000000002998. Epub 2023 Aug 2. Pain. 2024. PMID: 37530676
-
The Patient-Reported Outcomes Measurement Information System in spine surgery: a systematic review.J Neurosurg Spine. 2019 Jan 4;30(3):405-413. doi: 10.3171/2018.8.SPINE18608. Print 2019 Mar 1. J Neurosurg Spine. 2019. PMID: 30611150
Cited by
-
Using prior information to individualize start item selection when assessing physical functioning with the EORTC CAT Core.Health Qual Life Outcomes. 2025 Mar 9;23(1):21. doi: 10.1186/s12955-025-02353-3. Health Qual Life Outcomes. 2025. PMID: 40059213 Free PMC article.
-
Efficiency, Precision, Validity, and Reliability of GlauCAT-Asian Computerized Adaptive Tests in Measuring Glaucoma-Related Quality of Life.Transl Vis Sci Technol. 2024 Feb 1;13(2):6. doi: 10.1167/tvst.13.2.6. Transl Vis Sci Technol. 2024. PMID: 38329749 Free PMC article.
-
Psychometrics of patient-reported outcomes measurement information system in von Willebrand disease, inherited platelet function disorders, and rare bleeding disorders.Res Pract Thromb Haemost. 2024 Jun 13;8(4):102474. doi: 10.1016/j.rpth.2024.102474. eCollection 2024 May. Res Pract Thromb Haemost. 2024. PMID: 39076727 Free PMC article.
-
Patient-reported outcomes for people with diabetes: what and how to measure? A narrative review.Diabetologia. 2023 Aug;66(8):1357-1377. doi: 10.1007/s00125-023-05926-3. Epub 2023 May 24. Diabetologia. 2023. PMID: 37222772 Free PMC article. Review.
-
Associations of Pretransplant Patient-Reported Outcomes Measurement Information System Physical Function Score With Kidney Transplant Outcomes.Transpl Int. 2025 Jan 29;38:13884. doi: 10.3389/ti.2025.13884. eCollection 2025. Transpl Int. 2025. PMID: 39944218 Free PMC article.
References
-
- Almutary H, Bonner A, Douglas C.. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care 2013;39:140–50. https://doi.org.10.1111/j.1755-6686.2013.12022.x - PubMed
-
- Voskamp PWM, van Diepen M, Evans Met al. . The impact of symptoms on health-related quality of life in elderly pre-dialysis patients: effect and importance in the EQUAL study. Nephrol Dial Transplant 2019;34:1707–15. https://doi.org.10.1093/ndt/gfy167 - PubMed
-
- van der Willik EM, Hemmelder MH, Bart HAJet al. . Routinely measuring symptom burden and health-related quality of life in dialysis patients: first results from the Dutch registry of patient-reported outcome measures. Clin Kidney J 2021;14:1535–44. https://doi.org.10.1093/ckj/sfz192 - PMC - PubMed
-
- Raj R, Ahuja KD, Frandsen Met al. . Symptoms and their recognition in adult haemodialysis patients: interactions with quality of life. Nephrology (Carlton) 2017;22:228–33. https://doi.org.10.1111/nep.12754 - PubMed
-
- Manns B, Hemmelgarn B, Lillie Eet al. . Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 2014;9:1813–21. https://doi.org.10.2215/CJN.01610214 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

