Effects of Neural Mobilization on Sensory Dysfunction and Peripheral Nerve Degeneration in Rats With Painful Diabetic Neuropathy
- PMID: 35913760
- PMCID: PMC7613682
- DOI: 10.1093/ptj/pzac104
Effects of Neural Mobilization on Sensory Dysfunction and Peripheral Nerve Degeneration in Rats With Painful Diabetic Neuropathy
Abstract
Objective: This study aims to evaluate the effectiveness of neural mobilization (NM) in the management of sensory dysfunction and nerve degeneration related to experimental painful diabetic neuropathy (PDN).
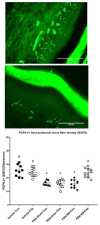
Methods: This is a pre-clinical animal study performed in the streptozocin-induced diabetic rat model. Three groups were included: a treatment group of rats with PDN receiving NM under anesthesia (PDN-NM, n = 10), a sham treatment group of rats with PDN that received only anesthesia (PDN-Sham, n = 9), and a vehicle control group with nondiabetic animals (Vehicle, n = 10). Rats in the PDN-NM and PDN-Sham groups received 1 treatment session on days 10, 12, and 14 after streptozocin injection, with a 48-hour rest period between sessions. Behavioral tests were performed using von Frey and Plantar tests. Evaluation for peripheral nerve degeneration was performed through measuring protein gene product 9.5-positive intra-epidermal nerve fiber density in hind-paw skin biopsies. All measurements were performed by a blinded investigator.
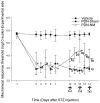
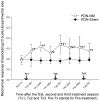
Results: The behavioral tests showed that a single NM session could reduce hyperalgesia, which was maintained for 48 hours. The second treatment session further improved this treatment effect, and the third session maintained it. These results suggest that it requires multiple treatment sessions to produce and maintain hypoalgesic effects. Skin biopsy analysis showed that the protein gene product 9.5-positive intra-epidermal nerve fiber density was higher on the experimental side of the PDN-NM group compared with the PDN-Sham group, suggesting NM may mitigate the degeneration of peripheral nerves.
Conclusion: This study demonstrated that NM may be an effective method to manage experimentally induced PDN, potentially through mitigation of nerve degeneration. Further studies are needed to develop standardized protocols for clinical use.
Impact: These findings provide neurophysiological evidence for the use of NM in PDN and can form the basis for the development of physical therapy-based programs in clinics.
Keywords: Diabetic Neuropathies; Nerve Degeneration; Pain Management.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Physical Therapy Association. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
Figures






Similar articles
-
Swimming exercise attenuates mechanical hypersensitivity and mitigates peripheral nerve degeneration in rats with painful diabetic neuropathy (PDN).Neurosci Lett. 2023 Aug 24;812:137406. doi: 10.1016/j.neulet.2023.137406. Epub 2023 Jul 21. Neurosci Lett. 2023. PMID: 37480979
-
Neural Mobilization Attenuates Mechanical Allodynia and Decreases Proinflammatory Cytokine Concentrations in Rats With Painful Diabetic Neuropathy.Phys Ther. 2018 Apr 1;98(4):214-222. doi: 10.1093/ptj/pzx124. Phys Ther. 2018. PMID: 29309710
-
10 kHz spinal cord stimulation improves metrics of spinal sensory processing in a male STZ rat model of diabetes.Neurosci Lett. 2024 Nov 1;842:137990. doi: 10.1016/j.neulet.2024.137990. Epub 2024 Sep 14. Neurosci Lett. 2024. PMID: 39278460
-
Advances in Interventional Therapies for Painful Diabetic Neuropathy: A Systematic Review.Anesth Analg. 2022 Jun 1;134(6):1215-1228. doi: 10.1213/ANE.0000000000005860. Epub 2022 Jan 20. Anesth Analg. 2022. PMID: 35051958 Free PMC article.
-
Diabetic Neuropathy Pain Management: A Global Challenge.Curr Diabetes Rev. 2021;17(5):e031120187542. doi: 10.2174/1573399816666201103142521. Curr Diabetes Rev. 2021. PMID: 33143631 Review.
Cited by
-
Mechanisms of neurodynamic treatments (MONET): a protocol for a mechanistic, randomised, single-blind controlled trial in patients with carpal tunnel syndrome.BMC Musculoskelet Disord. 2024 Jul 27;25(1):590. doi: 10.1186/s12891-024-07713-6. BMC Musculoskelet Disord. 2024. PMID: 39068435 Free PMC article.
-
Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions.MedComm (2020). 2024 Apr 12;5(4):e516. doi: 10.1002/mco2.516. eCollection 2024 Apr. MedComm (2020). 2024. PMID: 38617433 Free PMC article. Review.
-
Neuromodulation Through Magnetic Fields Irradiation with AT-04 Improves Hyperalgesia in a Rat Model of Neuropathic Pain via Descending Pain Modulatory Systems and Opioid Analgesia.Cell Mol Neurobiol. 2023 Nov;43(8):4345-4362. doi: 10.1007/s10571-023-01430-9. Epub 2023 Nov 7. Cell Mol Neurobiol. 2023. PMID: 37934363 Free PMC article.
References
-
- Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522. - PubMed
-
- Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91:733–737. - PubMed
-
- Calcutt NA, Backonja MM. Pathogenesis of pain in peripheral diabetic neuropathy. Curr Diab Rep. 2007;7:429–434. - PubMed
-
- Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22:1029–1035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

