Newly identified disorder of copper metabolism caused by variants in CTR1, a high-affinity copper transporter
- PMID: 35913762
- PMCID: PMC9759326
- DOI: 10.1093/hmg/ddac156
Newly identified disorder of copper metabolism caused by variants in CTR1, a high-affinity copper transporter
Abstract
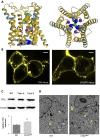
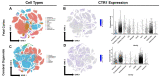
The high-affinity copper transporter CTR1 is encoded by CTR1 (SLC31A1), a gene locus for which no detailed genotype-phenotype correlations have previously been reported. We describe identical twin male infants homozygous for a novel missense variant NM_001859.4:c.284 G > A (p.Arg95His) in CTR1 with a distinctive autosomal recessive syndrome of infantile seizures and neurodegeneration, consistent with profound central nervous system copper deficiency. We used clinical, biochemical and molecular methods to delineate the first recognized examples of human CTR1 deficiency. These included clinical phenotyping, brain imaging, assays for copper, cytochrome c oxidase (CCO), and mitochondrial respiration, western blotting, cell transfection experiments, confocal and electron microscopy, protein structure modeling and fetal brain and cerebral organoid CTR1 transcriptome analyses. Comparison with two other critical mediators of cellular copper homeostasis, ATP7A and ATP7B, genes associated with Menkes disease and Wilson disease, respectively, revealed that expression of CTR1 was highest. Transcriptome analyses identified excitatory neurons and radial glia as brain cell types particularly enriched for copper transporter transcripts. We also assessed the effects of Copper Histidinate in the patients' cultured cells and in the patients, under a formal clinical protocol. Treatment normalized CCO activity and enhanced mitochondrial respiration in vitro, and was associated with modest clinical improvements. In combination with present and prior studies, these infants' clinical, biochemical and molecular phenotypes establish the impact of this novel variant on copper metabolism and cellular homeostasis and illuminate a crucial role for CTR1 in human brain development. CTR1 deficiency represents a newly defined inherited disorder of brain copper metabolism.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Inesi, G. (2017) Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life, 69, 211–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

