Spatial Organization of Dectin-1 and TLR2 during Synergistic Crosstalk Revealed by Super-resolution Imaging
- PMID: 35913832
- PMCID: PMC10636754
- DOI: 10.1021/acs.jpcb.2c03557
Spatial Organization of Dectin-1 and TLR2 during Synergistic Crosstalk Revealed by Super-resolution Imaging
Abstract
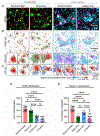
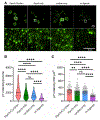
Innate immune cells recognize and elicit responses against pathogens by integrating signals from different types of cell-surface receptors. How the receptors interact in the membrane to enable their signaling crosstalk is poorly understood. Here, we reveal the nanoscale organization of TLR2 and Dectin-1, a receptor pair known to cooperate in regulating antifungal immunity, through their synergistic signaling crosstalk at macrophage cell membranes. Using super-resolution single-molecule localization microscopy, we show that discrete noncolocalized nanoclusters of Dectin-1 and TLR2 are partially overlapped during their synergistic crosstalk. Compared to when one type of receptor is activated alone, the simultaneous activation of Dectin-1 and TLR2 leads to a higher percentage of both receptors being activated by their specific ligands and consequently an increased level of tyrosine phosphorylation. Our results depict, in nanoscale detail, how Dectin-1 and TLR2 achieve synergistic signaling through the spatial organization of their receptor nanoclusters.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Thaiss CA; Levy M; Itav S; Elinav E Integration of Innate Immune Signaling. Trends Immunol. 2016, 37, 84–101. - PubMed
-
- van Egmond M; Vidarsson G; Bakema JE Cross-Talk between Pathogen Recognizing Toll-Like Receptors and Immunoglobulin Fc Receptors in Immunity. Immunol. Rev 2015, 268, 311–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

