Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge
- PMID: 35913836
- PMCID: PMC9384598
- DOI: 10.1093/jac/dkac256
Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge
Abstract
Background: Remdesivir and sotrovimab both have clinical trial data in the outpatient setting demonstrating reduction in the risk of hospitalizations and emergency department (ED) visits related to COVID-19.
Objectives: To evaluate the effectiveness of remdesivir in comparison with sotrovimab and matched high-risk control patients in preventing COVID-19-related hospitalizations and ED visits during the Omicron B.1.1.529 surge.
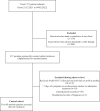
Patients and methods: This retrospective cohort study included outpatients positive for SARS-CoV-2, with non-severe symptoms for ≤7 days and deemed high-risk for severe COVID-19 by an internal scoring matrix. Patients who received remdesivir or sotrovimab from 27/12/2021 to 04/02/2022 were included (n = 82 and n = 88, respectively). These were compared with a control cohort of high-risk COVID-19 outpatients who did not receive therapy (n = 90). The primary outcome was a composite of 29 day COVID-19-related hospitalizations and/or ED visits. Pre-specified secondary outcomes included components of the primary endpoint, 29 day all-cause mortality and serious adverse drug events.
Results: Patients treated with remdesivir were significantly less likely to be hospitalized or visit the ED within 29 days from symptom onset (11% versus 23.3%; OR = 0.41, 95% CI = 0.17-0.95). Patients receiving sotrovimab were also less likely to be hospitalized or visit the ED (8% versus 23.3%; OR = 0.28, 95% CI = 0.11-0.71). There was no difference in the incidence of hospitalizations/ED visits between sotrovimab and remdesivir.
Conclusions: Our highest-risk outpatients with Omicron-related COVID-19 who received early sotrovimab or remdesivir had significantly lower likelihoods of a hospitalization and/or ED visit.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Johns Hopkins University . COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html.
-
- CDC . Underlying Medical Conditions Associated With Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Updated 15 June 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingco....
-
- Yek C, Warner S, Wiltz J et al. Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series - 465 health care facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep 2022; 71: 19–25. 10.15585/mmwr.mm7101a4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous