Safety and efficacy of a biodegradable implant releasing tenofovir alafenamide for vaginal protection in a macaque model
- PMID: 35913838
- PMCID: PMC10205616
- DOI: 10.1093/jac/dkac252
Safety and efficacy of a biodegradable implant releasing tenofovir alafenamide for vaginal protection in a macaque model
Abstract
Objectives: To advance the initiative of ending the global epidemic, long-lasting HIV protection is needed through sustained release of antiretroviral drugs for months to years. We investigated in macaques the safety and efficacy of biodegradable polycaprolactone implants releasing tenofovir alafenamide for HIV pre-exposure prophylaxis (PrEP).
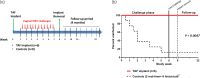
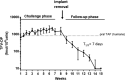
Methods: Implants were administered subcutaneously in the arm using a contraceptive trocar. Efficacy against vaginal simian-HIV (SHIV) infection was investigated in six pigtailed macaques that received two tenofovir alafenamide implants (0.35 mg/day), one in each arm, for a total release rate of tenofovir alafenamide at 0.7 mg/day. Macaques were exposed to SHIV twice weekly for 6 weeks. Statistical analyses were used to compare outcome with eight untreated controls. Histological assessments were performed on skin biopsies collected near implantation sites.
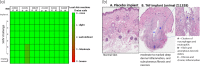
Results: Median (range) tenofovir diphosphate level in PBMCs was 1519 (1068-1898) fmol/106 cells. All macaques with tenofovir alafenamide implants were protected against vaginal SHIV infection. In contrast, 7/8 controls were infected after a median of 4 SHIV exposures (P = 0.0047). Histological assessment of tissues near tenofovir alafenamide implant sites showed inflammation and necrosis in 5/6 animals, which were not evident by visual inspection.
Conclusions: We demonstrated complete protection against vaginal SHIV infection with two implants releasing a total of 0.7 mg of tenofovir alafenamide per day. We also identified tenofovir diphosphate concentrations in PBMCs associated with complete vaginal protection. Consistent with previous findings, we observed adverse local toxicity and necrosis near the tenofovir alafenamide implant site. Improved tenofovir alafenamide implants that are safe and maintain high efficacy have the potential to provide long-lasting protection against vaginal HIV infection.
Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy 2022.
Figures
Similar articles
-
Efficacy of Oral Tenofovir Alafenamide/Emtricitabine Combination or Single-Agent Tenofovir Alafenamide Against Vaginal Simian Human Immunodeficiency Virus Infection in Macaques.J Infect Dis. 2019 Oct 22;220(11):1826-1833. doi: 10.1093/infdis/jiz383. J Infect Dis. 2019. PMID: 31362305
-
Single dose topical inserts containing tenofovir alafenamide fumarate and elvitegravir provide pre- and post-exposure protection against vaginal SHIV infection in macaques.EBioMedicine. 2022 Dec;86:104361. doi: 10.1016/j.ebiom.2022.104361. Epub 2022 Nov 21. EBioMedicine. 2022. PMID: 36423375 Free PMC article.
-
Two-dose emtricitabine/tenofovir alafenamide plus bictegravir prophylaxis protects macaques against SHIV infection.J Antimicrob Chemother. 2021 Feb 11;76(3):692-698. doi: 10.1093/jac/dkaa476. J Antimicrob Chemother. 2021. PMID: 33202006 Free PMC article.
-
The predictive value of macaque models of preexposure prophylaxis for HIV prevention.Curr Opin HIV AIDS. 2022 Jul 1;17(4):179-185. doi: 10.1097/COH.0000000000000738. Curr Opin HIV AIDS. 2022. PMID: 35762371 Free PMC article. Review.
-
Evaluating the combination of emtricitabine/ tenofovir alafenamide fumarate to reduce the risk of sexually acquired HIV-1-infection in at-risk adults.Expert Opin Pharmacother. 2021 Jul;22(10):1245-1251. doi: 10.1080/14656566.2021.1902504. Epub 2021 Apr 1. Expert Opin Pharmacother. 2021. PMID: 33691554 Review.
Cited by
-
An Ultra-Long-Acting Dimeric Bictegravir Prodrug Defined by a Short Pharmacokinetic Tail.Res Sq [Preprint]. 2025 Feb 19:rs.3.rs-5959131. doi: 10.21203/rs.3.rs-5959131/v1. Res Sq. 2025. PMID: 40034436 Free PMC article. Preprint.
-
Willingness of adolescent girls and young women to participate in future clinical trials of long-acting PrEP implants for HIV prevention, Kampala Uganda.PLOS Glob Public Health. 2025 Aug 8;5(8):e0005028. doi: 10.1371/journal.pgph.0005028. eCollection 2025. PLOS Glob Public Health. 2025. PMID: 40779595 Free PMC article.
-
REverse transcriptase ACTivity (REACT) assay for point-of-care measurement of established and emerging antiretrovirals for HIV treatment and prevention.Anal Bioanal Chem. 2024 Dec;416(29):6809-6818. doi: 10.1007/s00216-024-05602-4. Epub 2024 Oct 28. Anal Bioanal Chem. 2024. PMID: 39466376
-
A Bilayer Microarray Patch (MAP) for HIV Pre-Exposure Prophylaxis: The Role of MAP Designs and Formulation Composition in Enhancing Long-Acting Drug Delivery.Pharmaceutics. 2024 Jan 20;16(1):142. doi: 10.3390/pharmaceutics16010142. Pharmaceutics. 2024. PMID: 38276512 Free PMC article.
-
Zero-Order Kinetics Release of Lamivudine from Layer-by-Layer Coated Macromolecular Prodrug Particles.Int J Mol Sci. 2024 Dec 1;25(23):12921. doi: 10.3390/ijms252312921. Int J Mol Sci. 2024. PMID: 39684632 Free PMC article.
References
-
- UNAIDS . UNAIDS data 2019. 2019.. https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data.
-
- UNAIDS . Global HIV & AIDS statistics—2021 Fact sheet. 2021. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_....
-
- Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

