Examination of a nutritional treatment pathway according to pretreatment health status and stress levels of patients undergoing hematopoietic stem cell transplantation
- PMID: 35913908
- PMCID: PMC9342724
- DOI: 10.1371/journal.pone.0271728
Examination of a nutritional treatment pathway according to pretreatment health status and stress levels of patients undergoing hematopoietic stem cell transplantation
Abstract
Introduction: This study aimed to validate hematopoietic stem cell transplantation (HSCT) treatment via a tailored nutritional pathway in myeloablative conditioning (MAC), determine its efficacy in terms of remission, and explore associations between clinical outcomes and nutritional indicators.
Methods: We included patients who underwent MAC for HSCT at the Shizuoka Cancer Center Stem Cell Transplantation between 2015 and 2019. We evaluated outcomes from the day before treatment initiation (transplant date: day 0) to day 42.
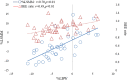
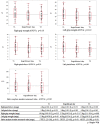
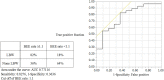
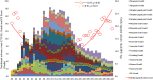
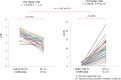
Results: Among the 40 MAC cases (participant characteristics: 20/40 males, mean age of 52 years, and mean body mass index of 21.9 kg/m2), we found that the percent loss of body weight and loss of skeletal muscle mass were correlated with the basal energy expenditure rate (BEE rate; r = 0.70, p<0.001 and r = 0.49, p<0.01, respectively). Based on the receiver operating characteristics curves, the cutoff value for the BEE rate in terms of weight loss was 1.1. Salivary amylase levels did not significantly change during the treatment course. Continuous variables, including oral caloric intake and performance status, showed statistically significant correlations with nutrition-related adverse events during treatment (r = -0.93, p<0.01 and r = 0.91, p<0.01, respectively). Skeletal muscle mass before treatment initiation was an independent predictive variable for reduced 2-year survival (p = 0.04).
Conclusion: Our results support the validity of a safe nutritional pathway with a BEE rate of 1.1 for HSCT patients pretreated with MAC. Specifically, we found that this pathway could prevent weight loss in response to nutrition-related adverse events. Skeletal muscle mass before treatment was identified as an independent risk factor for reduced 2-year survival.
Conflict of interest statement
No.
Figures



Similar articles
-
Benefit of Reducing Body Weight Loss with A Nutritional Support Pathway in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation.Med Sci Monit Basic Res. 2019 Sep 10;25:187-198. doi: 10.12659/MSMBR.917329. Med Sci Monit Basic Res. 2019. PMID: 31503241 Free PMC article.
-
Nutritional risk in allogeneic stem cell transplantation: rationale for a tailored nutritional pathway.Ann Hematol. 2017 Apr;96(4):617-625. doi: 10.1007/s00277-016-2910-9. Epub 2017 Jan 3. Ann Hematol. 2017. PMID: 28050676
-
Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic hematopoietic stem cell transplantation for acute leukemia patients: a single center experience.Transfus Apher Sci. 2013 Dec;49(3):590-9. doi: 10.1016/j.transci.2013.07.030. Epub 2013 Aug 8. Transfus Apher Sci. 2013. PMID: 23981652
-
Nutritional status and body mass index before hematopoietic stem cell transplantation (HSCT) and associated outcomes: a rapid review.Support Care Cancer. 2023 Dec 22;32(1):50. doi: 10.1007/s00520-023-08238-9. Support Care Cancer. 2023. PMID: 38129689 Free PMC article. Review.
-
Brazilian Nutritional Consensus in Hematopoietic Stem Cell Transplantation: Adults.Einstein (Sao Paulo). 2020 Feb 7;18:AE4530. doi: 10.31744/einstein_journal/2020AE4530. eCollection 2020. Einstein (Sao Paulo). 2020. PMID: 32049129 Free PMC article.
Cited by
-
The effect of oral nutrition supplement (ONS) on the nutritional and clinical status of patients undergoing autologous hematopoietic stem cell transplantation: study protocol for a randomized controlled clinical trial.BMC Nutr. 2024 Jun 10;10(1):83. doi: 10.1186/s40795-024-00893-3. BMC Nutr. 2024. PMID: 38858716 Free PMC article.
-
The Critical Exploration into Current Evidence behind the Role of the Nutritional Support in Adult Patients Who Undergo Haematogenic Stem Cell Transplantation.Nutrients. 2023 Aug 11;15(16):3558. doi: 10.3390/nu15163558. Nutrients. 2023. PMID: 37630748 Free PMC article. Review.
-
Relationship Between Preoperative Nutritional Indices and Sarcopenia in Patients With Stage III Colorectal Cancer.Nutr Metab Insights. 2022 Oct 14;15:11786388221129011. doi: 10.1177/11786388221129011. eCollection 2022. Nutr Metab Insights. 2022. PMID: 36263232 Free PMC article.
References
-
- The Japanese Data Center for Hematopoietic Cell Transplantation. Activities and outcomes of hematopoietic cell transplantation in Japan; 2018. [Cited 14 June 2021]. https://www.jdchct.or.jp/en/data/slide/2018/.
-
- Falda M, Busca A, Baldi I, Mordini N, Bruno B, Allione B, et al.. Nonmyeloablative allogeneic stem cell transplantation in elderly patients with hematological malignancies: Results from the GITMO (Gruppo Italiano Trapianto Midollo Osseo) multicenter prospective clinical trial. Am J Hematol. 2007;82: 863–866. doi: 10.1002/ajh.20990 - DOI - PubMed
-
- Ringdén O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al.. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27: 4570–4577. doi: 10.1200/JCO.2008.20.9692 - DOI - PubMed
-
- Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL, et al.. Clofarabine ± fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011;17: 893–900. doi: 10.1016/j.bbmt.2010.09.022 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

