Equine keratinocytes in the pathogenesis of insect bite hypersensitivity: Just another brick in the wall?
- PMID: 35913947
- PMCID: PMC9342730
- DOI: 10.1371/journal.pone.0266263
Equine keratinocytes in the pathogenesis of insect bite hypersensitivity: Just another brick in the wall?
Abstract
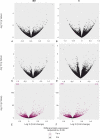
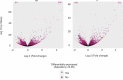
Equine insect bite hypersensitivity (IBH) is the most common skin disease affecting horses. It is described as an IgE-mediated, Type I hypersensitivity reaction to salivary gland proteins of Culicoides insects. Together with Th2 cells, epithelial barrier cells play an important role in development of Type I hypersensitivities. In order to elucidate the role of equine keratinocytes in development of IBH, we stimulated keratinocytes derived from IBH-affected (IBH-KER) (n = 9) and healthy horses (H-KER) (n = 9) with Culicoides recombinant allergens and extract, allergic cytokine milieu (ACM) and a Toll like receptor ligand 1/2 (TLR-1/2-L) and investigated their transcriptomes. Stimulation of keratinocytes with Culicoides allergens did not induce transcriptional changes. However, when stimulated with allergic cytokine milieu, their gene expression significantly changed. We found upregulation of genes encoding for CCL5, -11, -20, -27 and interleukins such as IL31. We also found a strong downregulation of genes such as SCEL and KRT16 involved in the formation of epithelial barrier. Following stimulation with TLR-1/2-L, keratinocytes significantly upregulated expression of genes affecting Toll like receptor and NOD-receptor signaling pathway as well as NF-kappa B signaling pathway, among others. The transcriptomes of IBH-KER and H-KER were very similar: without stimulations they only differed in one gene (CTSL); following stimulation with allergic cytokine milieu we found only 23 differentially expressed genes (e.g. CXCL10 and 11) and following stimulation with TLR-1/2-L they only differed by expression of seven genes. Our data suggests that keratinocytes contribute to the innate immune response and are able to elicit responses to different stimuli, possibly playing a role in the pathogenesis of IBH.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Investigating the epithelial barrier and immune signatures in the pathogenesis of equine insect bite hypersensitivity.PLoS One. 2020 Apr 28;15(4):e0232189. doi: 10.1371/journal.pone.0232189. eCollection 2020. PLoS One. 2020. PMID: 32343720 Free PMC article.
-
Equine insect bite hypersensitivity: immunoblot analysis of IgE and IgG subclass responses to Culicoides nubeculosus salivary gland extract.Vet Immunol Immunopathol. 2006 Sep 15;113(1-2):99-112. doi: 10.1016/j.vetimm.2006.04.009. Epub 2006 Jun 23. Vet Immunol Immunopathol. 2006. PMID: 16797724
-
Selective cloning, characterization, and production of the Culicoides nubeculosus salivary gland allergen repertoire associated with equine insect bite hypersensitivity.Vet Immunol Immunopathol. 2011 Feb 15;139(2-4):200-9. doi: 10.1016/j.vetimm.2010.10.015. Epub 2010 Oct 15. Vet Immunol Immunopathol. 2011. PMID: 21071100
-
Equine insect bite hypersensitivity: what do we know?Vet Immunol Immunopathol. 2012 Jun 30;147(3-4):113-26. doi: 10.1016/j.vetimm.2012.03.017. Epub 2012 Apr 3. Vet Immunol Immunopathol. 2012. PMID: 22575371 Review.
-
Equine allergic skin diseases: Clinical consensus guidelines of the World Association for Veterinary Dermatology.Vet Dermatol. 2023 Jun;34(3):175-208. doi: 10.1111/vde.13168. Vet Dermatol. 2023. PMID: 37154488 Review.
Cited by
-
Cytokines and chemokines skin gene expression in correlation with immune cells in blood and severity in equine insect bite hypersensitivity.Front Immunol. 2024 Jul 15;15:1414891. doi: 10.3389/fimmu.2024.1414891. eCollection 2024. Front Immunol. 2024. PMID: 39076967 Free PMC article.
-
Skin Barrier in Normal and Allergic Horses: What Do We Know?Vet Sci. 2025 Jan 24;12(2):91. doi: 10.3390/vetsci12020091. Vet Sci. 2025. PMID: 40005851 Free PMC article. Review.
-
Insect Bite Hypersensitivity in Horses: Causes, Diagnosis, Scoring and New Therapies.Animals (Basel). 2023 Aug 4;13(15):2514. doi: 10.3390/ani13152514. Animals (Basel). 2023. PMID: 37570323 Free PMC article. Review.
References
-
- Pilsworth RC, Knottenbelt DC. Equine insect hypersensitivity. Equine Veterinary Education. 2004;16(6):324–5. doi: 10.1111/j.2042-3292.2004.tb00321.x - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

