Chemical evidence for the tradeoff-in-the-nephron hypothesis to explain secondary hyperparathyroidism
- PMID: 35913960
- PMCID: PMC9342777
- DOI: 10.1371/journal.pone.0272380
Chemical evidence for the tradeoff-in-the-nephron hypothesis to explain secondary hyperparathyroidism
Abstract
Background: Secondary hyperparathyroidism (SHPT) complicates advanced chronic kidney disease (CKD) and causes skeletal and other morbidity. In animal models of CKD, SHPT was prevented and reversed by reduction of dietary phosphate in proportion to GFR, but the phenomena underlying these observations are not understood. The tradeoff-in-the-nephron hypothesis states that as GFR falls, the phosphate concentration in the distal convoluted tubule ([P]DCT]) rises, reduces the ionized calcium concentration in that segment ([Ca++]DCT), and thereby induces increased secretion of parathyroid hormone (PTH) to maintain normal calcium reabsorption. In patients with CKD, we previously documented correlations between [PTH] and phosphate excreted per volume of filtrate (EP/Ccr), a surrogate for [P]DCT. In the present investigation, we estimated [P]DCT from physiologic considerations and measurements of phosphaturia, and sought evidence for a specific chemical phenomenon by which increased [P]DCT could lower [Ca++]DCT and raise [PTH].
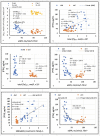
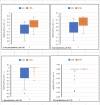
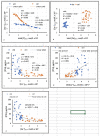
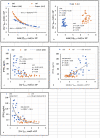
Methods and findings: We studied 28 patients ("CKD") with eGFR of 14-49 mL/min/1.73m2 (mean 29.9 ± 9.5) and 27 controls ("CTRL") with eGFR > 60 mL/min/1.73m2 (mean 86.2 ± 10.2). In each subject, total [Ca]DCT and [P]DCT were deduced from relevant laboratory data. The Joint Expert Speciation System (JESS) was used to calculate [Ca++]DCT and concentrations of related chemical species under the assumption that a solid phase of amorphous calcium phosphate (Ca3(PO4)2 (am., s.)) could precipitate. Regressions of [PTH] on eGFR, [P]DCT, and [Ca++]DCT were then examined. At filtrate pH of 6.8 and 7.0, [P]DCT was found to be the sole determinant of [Ca++]DCT, and precipitation of Ca3(PO4)2 (am., s.) appeared to mediate this result. At pH 6.6, total [Ca]DCT was the principal determinant of [Ca++]DCT, [P]DCT was a minor determinant, and precipitation of Ca3(PO4)2 (am., s.) was predicted in no CKD and five CTRL. In CKD, at all three pH values, [PTH] varied directly with [P]DCT and inversely with [Ca++]DCT, and a reduced [Ca++]DCT was identified at which [PTH] rose unequivocally. Relationships of [PTH] to [Ca++]DCT and to eGFR resembled each other closely.
Conclusions: As [P]DCT increases, chemical speciation calculations predict reduction of [Ca++]DCT through precipitation of Ca3(PO4)2 (am., s.). [PTH] appears to rise unequivocally if [Ca++]DCT falls sufficiently. These results support the tradeoff-in-the-nephron hypothesis, and they explain why proportional phosphate restriction prevented and reversed SHPT in experimental CKD. Whether equally stringent treatment can be as efficacious in humans warrants investigation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Tradeoff-in-the-Nephron: A Theory to Explain the Primacy of Phosphate in the Pathogenesis of Secondary Hyperparathyroidism.Nutrients. 2017 Apr 26;9(5):427. doi: 10.3390/nu9050427. Nutrients. 2017. PMID: 28445401 Free PMC article.
-
An examination of multiple explanations for secondary hyperparathyroidism .Clin Nephrol. 2020 Aug;94(2):70-77. doi: 10.5414/CN110043. Clin Nephrol. 2020. PMID: 32567541
-
Use of sevelamer to examine the role of intraluminal phosphate in the pathogenesis of secondary hyperparathyroidism.Clin Nephrol. 2014 Sep;82(3):191-201. doi: 10.5414/cn108227. Clin Nephrol. 2014. PMID: 25079864 Clinical Trial.
-
1alpha(OH)D3 One-alpha-hydroxy-cholecalciferol--an active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis.Dan Med Bull. 2008 Nov;55(4):186-210. Dan Med Bull. 2008. PMID: 19232159 Review.
-
Hyporesponsiveness or resistance to the action of parathyroid hormone in chronic kidney disease.Nefrologia (Engl Ed). 2021 Sep-Oct;41(5):514-528. doi: 10.1016/j.nefroe.2021.11.014. Epub 2021 Dec 14. Nefrologia (Engl Ed). 2021. PMID: 36165134 Review.
References
-
- Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al.. Prevalence of abnormal serum vitamin D, PTH, calcium and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007; 71: 31–38. doi: 10.1038/sj.ki.5002009 - DOI - PubMed
-
- Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high FGF23 levels of experimental kidney failure: a bone parathyroid hormone feedback loop. Am J Physiol Renal Physiol. 2010;299: F882–F889. doi: 10.1152/ajprenal.00360.2010 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

