PrrA modulates Mycobacterium tuberculosis response to multiple environmental cues and is critically regulated by serine/threonine protein kinases
- PMID: 35913986
- PMCID: PMC9371303
- DOI: 10.1371/journal.pgen.1010331
PrrA modulates Mycobacterium tuberculosis response to multiple environmental cues and is critically regulated by serine/threonine protein kinases
Abstract
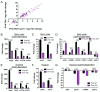
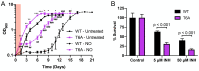
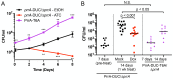
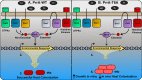
The ability of Mycobacterium tuberculosis (Mtb) to adapt to its surrounding environment is critical for the bacterium to successfully colonize its host. Transcriptional changes are a vital mechanism by which Mtb responds to key environmental signals experienced, such as pH, chloride (Cl-), nitric oxide (NO), and hypoxia. However, much remains unknown regarding how Mtb coordinates its response to the disparate signals seen during infection. Utilizing a transcription factor (TF) overexpression plasmid library in combination with a pH/Cl--responsive luciferase reporter, we identified the essential TF, PrrA, part of the PrrAB two-component system, as a TF involved in modulation of Mtb response to pH and Cl-. Further studies revealed that PrrA also affected Mtb response to NO and hypoxia, with prrA overexpression dampening induction of NO and hypoxia-responsive genes. PrrA is phosphorylated not just by its cognate sensor histidine kinase PrrB, but also by serine/threonine protein kinases (STPKs) at a second distinct site. Strikingly, a STPK-phosphoablative PrrA variant was significantly dampened in its response to NO versus wild type Mtb, disrupted in its ability to adaptively enter a non-replicative state upon extended NO exposure, and attenuated for in vivo colonization. Together, our results reveal PrrA as an important regulator of Mtb response to multiple environmental signals, and uncover a critical role of STPK regulation of PrrA in its function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
M. tuberculosis PrrA binds the dosR promoter and regulates mycobacterial adaptation to hypoxia.Tuberculosis (Edinb). 2024 Sep;148:102531. doi: 10.1016/j.tube.2024.102531. Epub 2024 Jun 8. Tuberculosis (Edinb). 2024. PMID: 38885567
-
The prrAB two-component system is essential for Mycobacterium tuberculosis viability and is induced under nitrogen-limiting conditions.J Bacteriol. 2012 Jan;194(2):354-61. doi: 10.1128/JB.06258-11. Epub 2011 Nov 11. J Bacteriol. 2012. PMID: 22081401 Free PMC article.
-
Mycobacterium tuberculosis PknK Substrate Profiling Reveals Essential Transcription Terminator Protein Rho and Two-Component Response Regulators PrrA and MtrA as Novel Targets for Phosphorylation.Microbiol Spectr. 2022 Apr 27;10(2):e0135421. doi: 10.1128/spectrum.01354-21. Epub 2022 Apr 11. Microbiol Spectr. 2022. PMID: 35404097 Free PMC article.
-
Mycobacterium tuberculosis Serine/Threonine Protein Kinases.Microbiol Spectr. 2014 Oct;2(5):10.1128/microbiolspec.MGM2-0006-2013. doi: 10.1128/microbiolspec.MGM2-0006-2013. Microbiol Spectr. 2014. PMID: 25429354 Free PMC article. Review.
-
An Overview on the Potential Antimycobacterial Agents Targeting Serine/Threonine Protein Kinases from Mycobacterium tuberculosis.Curr Top Med Chem. 2019;19(9):646-661. doi: 10.2174/1568026619666190227182701. Curr Top Med Chem. 2019. PMID: 30827246 Review.
Cited by
-
Mycobacterium tuberculosis response to cholesterol is integrated with environmental pH and potassium levels via a lipid metabolism regulator.PLoS Genet. 2024 Jan 24;20(1):e1011143. doi: 10.1371/journal.pgen.1011143. eCollection 2024 Jan. PLoS Genet. 2024. PMID: 38266039 Free PMC article.
-
Targeting Mycobacterium tuberculosis pH-driven adaptation.Microbiology (Reading). 2024 May;170(5):001458. doi: 10.1099/mic.0.001458. Microbiology (Reading). 2024. PMID: 38717801 Free PMC article. Review.
-
Clinically relevant mutations in the PhoR sensor kinase of host-adapted Mycobacterium abscessus isolates impact response to acidic pH and virulence.Microbiol Spectr. 2023 Dec 12;11(6):e0158823. doi: 10.1128/spectrum.01588-23. Epub 2023 Oct 24. Microbiol Spectr. 2023. PMID: 37874174 Free PMC article.
-
Comparison of the transcriptome, lipidome, and c-di-GMP production between BCGΔBCG1419c and BCG, with Mincle- and Myd88-dependent induction of proinflammatory cytokines in murine macrophages.Sci Rep. 2024 May 24;14(1):11898. doi: 10.1038/s41598-024-61815-8. Sci Rep. 2024. PMID: 38789479 Free PMC article.
-
Dual functioning by the PhoR sensor is a key determinant to Mycobacterium tuberculosis virulence.PLoS Genet. 2023 Dec 15;19(12):e1011070. doi: 10.1371/journal.pgen.1011070. eCollection 2023 Dec. PLoS Genet. 2023. PMID: 38100394 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

