T cells use distinct topographical and membrane receptor scanning strategies that individually coalesce during receptor recognition
- PMID: 35914144
- PMCID: PMC9372542
- DOI: 10.1073/pnas.2203247119
T cells use distinct topographical and membrane receptor scanning strategies that individually coalesce during receptor recognition
Abstract
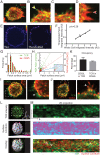
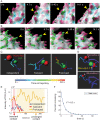
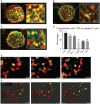
During immune surveillance, CD8 T cells scan the surface of antigen-presenting cells using dynamic microvillar palpation and movements as well as by having their receptors preconcentrated into patches. Here, we use real-time lattice light-sheet microscopy to demonstrate the independence of microvillar and membrane receptor patch scanning. While T cell receptor (TCR) patches can distribute to microvilli, they do so stochastically and not preferentially as for other receptors such as CD62L. The distinctness of TCR patch movement from microvillar movement extends to many other receptors that form patches that also scan independent of the TCR. An exception to this is the CD8 coreceptor which largely comigrates in patches that overlap with or are closely adjacent to those containing TCRs. Microvilli that assemble into a synapse contain various arrays of the engaged patches, notably of TCRs and the inhibitory receptor PD-1, creating a pastiche of occupancies that vary from microvillar contact to contact. In summary, this work demonstrates that localization of receptor patches within the membrane and on microvillar projections is random prior to antigen detection and that such random variation may play into the generation of many individually composed receptor patch compositions at a single synapse.
Keywords: T cell; T cell receptor; microvilli; synapse.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Davis M. M., et al. , T cells as a self-referential, sensory organ. Annu. Rev. Immunol. 25, 681–695 (2007). - PubMed
-
- van der Merwe P. A., Dushek O., Mechanisms for T cell receptor triggering. Nat. Rev. Immunol. 11, 47–55 (2011). - PubMed
-
- Grakoui A., et al. , The immunological synapse: A molecular machine controlling T cell activation. Science 285, 221–227 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

