Cell-penetrating peptides enhance peptide vaccine accumulation and persistence in lymph nodes to drive immunogenicity
- PMID: 35914154
- PMCID: PMC9371699
- DOI: 10.1073/pnas.2204078119
Cell-penetrating peptides enhance peptide vaccine accumulation and persistence in lymph nodes to drive immunogenicity
Abstract
Peptide-based cancer vaccines are widely investigated in the clinic but exhibit modest immunogenicity. One approach that has been explored to enhance peptide vaccine potency is covalent conjugation of antigens with cell-penetrating peptides (CPPs), linear cationic and amphiphilic peptide sequences designed to promote intracellular delivery of associated cargos. Antigen-CPPs have been reported to exhibit enhanced immunogenicity compared to free peptides, but their mechanisms of action in vivo are poorly understood. We tested eight previously described CPPs conjugated to antigens from multiple syngeneic murine tumor models and found that linkage to CPPs enhanced peptide vaccine potency in vivo by as much as 25-fold. Linkage of antigens to CPPs did not impact dendritic cell activation but did promote uptake of linked antigens by dendritic cells both in vitro and in vivo. However, T cell priming in vivo required Batf3-dependent dendritic cells, suggesting that antigens delivered by CPP peptides were predominantly presented via the process of cross-presentation and not through CPP-mediated cytosolic delivery of peptide to the classical MHC class I antigen processing pathway. Unexpectedly, we observed that many CPPs significantly enhanced antigen accumulation in draining lymph nodes. This effect was associated with the ability of CPPs to bind to lymph-trafficking lipoproteins and protection of CPP-antigens from proteolytic degradation in serum. These two effects resulted in prolonged presentation of CPP-peptides in draining lymph nodes, leading to robust T cell priming and expansion. Thus, CPPs can act through multiple unappreciated mechanisms to enhance T cell priming that can be exploited for cancer vaccines with enhanced potency.
Keywords: cancer immunotherapy; cell penetrating peptides; peptide vaccines.
Conflict of interest statement
Competing interest statement: B.L.P. is a cofounder and/or member of the scientific advisory board of several companies focusing on the development of protein and peptide therapeutics. C.J.W. is an equity holder of BioNTech, Inc. D.R. receives institutional support through Dana-Farber Cancer Institute from Acerta Phamaceuticals, Agenus, Bristol-Myers Squibb, Celldex, EMD Serono, Enterome, Epitopoietic Research Coorporation, Incyte, Inovio, Insightec, Novartis, Omniox, and Tragara; and is an advisor/consultant for Abbvie, Advantagene, Agenus, Agios, Amgen, AnHeart Therapeutics, Avita Biomedical, Bayer, Boston Biomedical, Boehringer Ingelheim, Bristol-Myers Squibb, Celldex, Deciphera, Del Mar Pharma, DNAtrix, Ellipses Pharma, EMD Serono, Genenta, Genentech/Roche, Hoffman-LaRoche, Imvax, Inovio, Kintara, Kiyatec, Medicenna Biopharma, Merck, Merck KGaA, Monteris, Neuvogen, Novartis, Novocure, Oncorus, Oxigene, Regeneron, Stemline, Sumitono Dainippon Pharma, Pyramid, Taiho Oncology, Vivacitas Oncology, and Y-mabs Therapeutics.
Figures


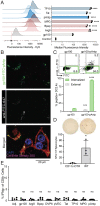
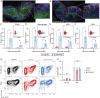
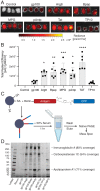

Similar articles
-
Enhancement of Peptide Vaccine Immunogenicity by Increasing Lymphatic Drainage and Boosting Serum Stability.Cancer Immunol Res. 2018 Sep;6(9):1025-1038. doi: 10.1158/2326-6066.CIR-17-0607. Epub 2018 Jun 18. Cancer Immunol Res. 2018. PMID: 29915023 Free PMC article.
-
No advantage of cell-penetrating peptides over receptor-specific antibodies in targeting antigen to human dendritic cells for cross-presentation.J Immunol. 2008 Jun 1;180(11):7687-96. doi: 10.4049/jimmunol.180.11.7687. J Immunol. 2008. PMID: 18490772
-
Induction of tumor-specific CTL responses using the C-terminal fragment of Viral protein R as cell penetrating peptide.Sci Rep. 2019 Mar 8;9(1):3937. doi: 10.1038/s41598-019-40594-7. Sci Rep. 2019. PMID: 30850685 Free PMC article.
-
Enhancing cancer immunotherapy by intracellular delivery of cell-penetrating peptides and stimulation of pattern-recognition receptor signaling.Adv Immunol. 2012;114:151-76. doi: 10.1016/B978-0-12-396548-6.00006-8. Adv Immunol. 2012. PMID: 22449781 Free PMC article. Review.
-
Use of Cell-Penetrating Peptides in Dendritic Cell-Based Vaccination.Immune Netw. 2016 Feb;16(1):33-43. doi: 10.4110/in.2016.16.1.33. Epub 2016 Feb 25. Immune Netw. 2016. PMID: 26937230 Free PMC article. Review.
Cited by
-
One Health Approach to the Computational Design of a Lipoprotein-Based Multi-Epitope Vaccine Against Human and Livestock Tuberculosis.Int J Mol Sci. 2025 Feb 13;26(4):1587. doi: 10.3390/ijms26041587. Int J Mol Sci. 2025. PMID: 40004053 Free PMC article.
-
Soluble antigen arrays provide increased efficacy and safety over free peptides for tolerogenic immunotherapy.Front Immunol. 2024 Jun 12;15:1258369. doi: 10.3389/fimmu.2024.1258369. eCollection 2024. Front Immunol. 2024. PMID: 38933266 Free PMC article.
-
Approaches for evaluation of novel CPP-based cargo delivery systems.Front Pharmacol. 2022 Oct 21;13:1056467. doi: 10.3389/fphar.2022.1056467. eCollection 2022. Front Pharmacol. 2022. PMID: 36339538 Free PMC article. Review.
-
Advances and prospects of cell-penetrating peptides in tumor immunotherapy.Sci Rep. 2025 Jan 27;15(1):3392. doi: 10.1038/s41598-025-86130-8. Sci Rep. 2025. PMID: 39870681 Free PMC article. Review.
-
Neoantigens: promising targets for cancer therapy.Signal Transduct Target Ther. 2023 Jan 6;8(1):9. doi: 10.1038/s41392-022-01270-x. Signal Transduct Target Ther. 2023. PMID: 36604431 Free PMC article. Review.
References
-
- Sahin U., Türeci Ö., Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018). - PubMed
-
- Hansen P. R., Oddo A., Peptide antibodies. Methods Mol. Biol. 1348, 33–50 (2015). - PubMed
-
- Winkler D. F. H., Automated solid-phase peptide synthesis. Methods Mol. Biol. 2103, 59–94 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

