Lymph-derived chemokines direct early neutrophil infiltration in the lymph nodes upon Staphylococcus aureus skin infection
- PMID: 35914162
- PMCID: PMC9371737
- DOI: 10.1073/pnas.2111726119
Lymph-derived chemokines direct early neutrophil infiltration in the lymph nodes upon Staphylococcus aureus skin infection
Abstract
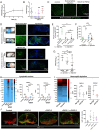
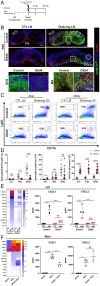
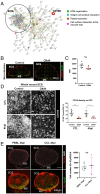
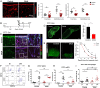
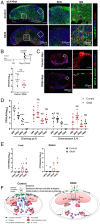
A large number of neutrophils infiltrate the lymph node (LN) within 4 h after Staphylococcus aureus skin infection (4 h postinfection [hpi]) and prevent systemic S. aureus dissemination. It is not clear how infection in the skin can remotely and effectively recruit neutrophils to the LN. Here, we found that lymphatic vessel occlusion substantially reduced neutrophil recruitment to the LN. Lymphatic vessels effectively transported bacteria and proinflammatory chemokines (i.e., Chemokine [C-X-C motif] motif 1 [CXCL1] and CXCL2) to the LN. However, in the absence of lymph flow, S. aureus alone in the LN was insufficient to recruit neutrophils to the LN at 4 hpi. Instead, lymph flow facilitated the earliest neutrophil recruitment to the LN by delivering chemokines (i.e., CXCL1, CXCL2) from the site of infection. Lymphatic dysfunction is often found during inflammation. During oxazolone (OX)-induced skin inflammation, CXCL1/2 in the LN was reduced after infection. The interrupted LN conduits further disrupted the flow of lymph and impeded its communication with high endothelial venules (HEVs), resulting in impaired neutrophil migration. The impaired neutrophil interaction with bacteria contributed to persistent infection in the LN. Our studies showed that both the flow of lymph from lymphatic vessels to the LN and the distribution of lymph in the LN are critical to ensure optimal neutrophil migration and timely innate immune protection in S. aureus infection.
Keywords: infection; lymph flow; lymph node; lymphatic vessel; neutrophil.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Kolaczkowska E., Kubes P., Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013). - PubMed
-
- Gerner M. Y., Torabi-Parizi P., Germain R. N., Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42, 172–185 (2015). - PubMed
-
- Qi H., Kastenmüller W., Germain R. N., Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu. Rev. Cell Dev. Biol. 30, 141–167 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

