Sulfur and methane oxidation by a single microorganism
- PMID: 35914169
- PMCID: PMC9371685
- DOI: 10.1073/pnas.2114799119
Sulfur and methane oxidation by a single microorganism
Abstract
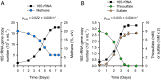
Natural and anthropogenic wetlands are major sources of the atmospheric greenhouse gas methane. Methane emissions from wetlands are mitigated by methanotrophic bacteria at the oxic-anoxic interface, a zone of intense redox cycling of carbon, sulfur, and nitrogen compounds. Here, we report on the isolation of an aerobic methanotrophic bacterium, 'Methylovirgula thiovorans' strain HY1, which possesses metabolic capabilities never before found in any methanotroph. Most notably, strain HY1 is the first bacterium shown to aerobically oxidize both methane and reduced sulfur compounds for growth. Genomic and proteomic analyses showed that soluble methane monooxygenase and XoxF-type alcohol dehydrogenases are responsible for methane and methanol oxidation, respectively. Various pathways for respiratory sulfur oxidation were present, including the Sox-rDsr pathway and the S4I system. Strain HY1 employed the Calvin-Benson-Bassham cycle for CO2 fixation during chemolithoautotrophic growth on reduced sulfur compounds. Proteomic and microrespirometry analyses showed that the metabolic pathways for methane and thiosulfate oxidation were induced in the presence of the respective substrates. Methane and thiosulfate could therefore be independently or simultaneously oxidized. The discovery of this versatile bacterium demonstrates that methanotrophy and thiotrophy are compatible in a single microorganism and underpins the intimate interactions of methane and sulfur cycles in oxic-anoxic interface environments.
Keywords: facultative methanotrophy; mixotrophy; thiotrophy; wetland.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Allen G., Biogeochemistry: Rebalancing the global methane budget. Nature 538, 46–48 (2016). - PubMed
-
- Saunois M., Jackson R. B., Bousquet P., Poulter B., Canadell J. G., The growing role of methane in anthropogenic climate change. Environ. Res. Lett. 11, 120207 (2016).
-
- Matthews E., “Wetlands” in Atmospheric Methane: Its Role in the Global Environment, Khalil M. A. K., Ed. (Springer Berlin Heidelberg, Berlin, 2000), pp. 202–233.
-
- Houghton J. T., et al. , Climate Change 2001: The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, Cambridge, UK, 2001).
-
- Conrad R., Rothfuss F., Methane oxidation in the soil surface layer of a flooded rice field and the effect of ammonium. Biol. Fertil. Soils 12, 28–32 (1991).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

