Radius measurement via super-resolution microscopy enables the development of a variable radii proximity labeling platform
- PMID: 35914173
- PMCID: PMC9371666
- DOI: 10.1073/pnas.2203027119
Radius measurement via super-resolution microscopy enables the development of a variable radii proximity labeling platform
Abstract
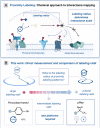
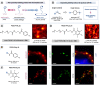
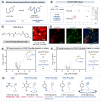
The elucidation of protein interaction networks is critical to understanding fundamental biology as well as developing new therapeutics. Proximity labeling platforms (PLPs) are state-of-the-art technologies that enable the discovery and delineation of biomolecular networks through the identification of protein-protein interactions. These platforms work via catalytic generation of reactive probes at a biological region of interest; these probes then diffuse through solution and covalently "tag" proximal biomolecules. The physical distance that the probes diffuse determines the effective labeling radius of the PLP and is a critical parameter that influences the scale and resolution of interactome mapping. As such, by expanding the degrees of labeling resolution offered by PLPs, it is possible to better capture the various size scales of interactomes. At present, however, there is little quantitative understanding of the labeling radii of different PLPs. Here, we report the development of a superresolution microscopy-based assay for the direct quantification of PLP labeling radii. Using this assay, we provide direct extracellular measurements of the labeling radii of state-of-the-art antibody-targeted PLPs, including the peroxidase-based phenoxy radical platform (269 ± 41 nm) and the high-resolution iridium-catalyzed µMap technology (54 ± 12 nm). Last, we apply these insights to the development of a molecular diffusion-based approach to tuning PLP resolution and introduce a new aryl-azide-based µMap platform with an intermediate labeling radius (80 ± 28 nm).
Keywords: STED microscopy; photoredox catalysis; proximity labeling.
Conflict of interest statement
Competing interest statement: A provisional U.S. patent has been filed by D.W.C.M. and C.P.S. based in part on this work, 62/982,366; 63/076,658. International Application No. PCT/US2021/019959. D.W.C.M. declares an ownership interest, C.P.S. declares an affiliation interest, in the company Dexterity Pharma LLC, which has commercialized materials used in this work. D.W.C.M. declares an ownership interest in PennPhD, which has commercialized materials used in this work.
Figures

References
-
- Baumeister S. H., Freeman G. J., Dranoff G., Sharpe A. H., Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 34, 539–573 (2016). - PubMed
-
- Scott D. E., Bayly A. R., Abell C., Skidmore J., Small molecules, big targets: Drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 15, 533–550 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

