MRI-Based Digital Models Forecast Patient-Specific Treatment Responses to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer
- PMID: 35914239
- PMCID: PMC9481712
- DOI: 10.1158/0008-5472.CAN-22-1329
MRI-Based Digital Models Forecast Patient-Specific Treatment Responses to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer
Abstract
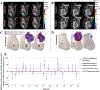
Triple-negative breast cancer (TNBC) is persistently refractory to therapy, and methods to improve targeting and evaluation of responses to therapy in this disease are needed. Here, we integrate quantitative MRI data with biologically based mathematical modeling to accurately predict the response of TNBC to neoadjuvant systemic therapy (NAST) on an individual basis. Specifically, 56 patients with TNBC enrolled in the ARTEMIS trial (NCT02276443) underwent standard-of-care doxorubicin/cyclophosphamide (A/C) and then paclitaxel for NAST, where dynamic contrast-enhanced MRI and diffusion-weighted MRI were acquired before treatment and after two and four cycles of A/C. A biologically based model was established to characterize tumor cell movement, proliferation, and treatment-induced cell death. Two evaluation frameworks were investigated using: (i) images acquired before and after two cycles of A/C for calibration and predicting tumor status after A/C, and (ii) images acquired before, after two cycles, and after four cycles of A/C for calibration and predicting response following NAST. For Framework 1, the concordance correlation coefficients between the predicted and measured patient-specific, post-A/C changes in tumor cellularity and volume were 0.95 and 0.94, respectively. For Framework 2, the biologically based model achieved an area under the receiver operator characteristic curve of 0.89 (sensitivity/specificity = 0.72/0.95) for differentiating pathological complete response (pCR) from non-pCR, which is statistically superior (P < 0.05) to the value of 0.78 (sensitivity/specificity = 0.72/0.79) achieved by tumor volume measured after four cycles of A/C. Overall, this model successfully captured patient-specific, spatiotemporal dynamics of TNBC response to NAST, providing highly accurate predictions of NAST response.
Significance: Integrating MRI data with biologically based mathematical modeling successfully predicts breast cancer response to chemotherapy, suggesting digital twins could facilitate a paradigm shift from simply assessing response to predicting and optimizing therapeutic efficacy.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Liu SV, Melstrom L, Yao K, Russell CA, Sener SF. Neoadjuvant therapy for breast cancer. J Surg Oncol 2010;101:283–291. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–1281. - PubMed
-
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379(22):2108–2121. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

