CD44 receptor targeted nanoparticles augment immunity against tuberculosis in mice
- PMID: 35914613
- PMCID: PMC10478167
- DOI: 10.1016/j.jconrel.2022.07.040
CD44 receptor targeted nanoparticles augment immunity against tuberculosis in mice
Abstract
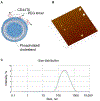
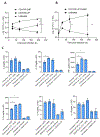
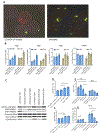
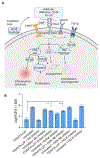
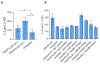
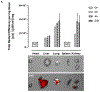
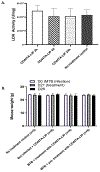
We describe a role of CD44-mediated signaling during host-defense against tuberculosis (TB) using a mouse model of TB and studies in M. tuberculosis (Mtb) infected human macrophage (MФ). Liposomes targeting CD44 using thioaptamers (CD44TA-LIP) were designed and tested as new vaccines to boost host immunity in TB. CD44TA-LIP enhanced killing of Mtb in human MФ, which correlated with an increased production of pro-inflammatory cytokines IL-1β, TNF-α and IL-12. CD44TA-LIP activated MФ showed an enhanced MHC-II dependent antigen presentation to CD4 T-cells. Inhibition of cellular proliferation and cytoskeleton rearrangement pathways downstream of CD44 signaling abrogated CD44TA-LIP-induced antimicrobial effects. Blockade of inflammatory pathways also reduced antigen presentation by MФ and activation of CD4 T cells. Mtb infected MФ treated with CD44TA-LIP exhibited increased nitric oxide and HβD2 defensin peptide production. Among Mtb infected mice with increased lung and spleen loads of organisms, intranasal administration of CD44TA-LIP led to a ten-fold reduction of colony forming units of Mtb and elevated IFN-γ + CD4, effector, central and resident memory T cells. Biodistribution studies demonstrated that CD44TA-LIP preferentially accumulated in the lungs and were associated with CD11b + cells. CD44TA-LIP treated mice showed no weight loss or increased liver LDH levels. This study highlights the importance of CD44-mediated signaling in host-defense during TB and the therapeutic potential of CD44TA-LIP.
Keywords: CD4 T cells; CD44; Host-defense; Host-directed therapy; Immunity; Liposomes; Macrophages; Mycobacterium tuberculosis; Thioaptamers; Tuberculosis.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures



Similar articles
-
Thioaptamer targeted discoidal microparticles increase self immunity and reduce Mycobacterium tuberculosis burden in mice.J Control Release. 2017 Nov 28;266:238-247. doi: 10.1016/j.jconrel.2017.09.038. Epub 2017 Oct 4. J Control Release. 2017. PMID: 28987879
-
Human M1 macrophages express unique innate immune response genes after mycobacterial infection to defend against tuberculosis.Commun Biol. 2022 May 19;5(1):480. doi: 10.1038/s42003-022-03387-9. Commun Biol. 2022. PMID: 35590096 Free PMC article.
-
Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen.Int Immunopharmacol. 2010 Jun;10(6):694-706. doi: 10.1016/j.intimp.2010.03.014. Epub 2010 Apr 7. Int Immunopharmacol. 2010. PMID: 20381647
-
Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis.Front Immunol. 2014 Apr 22;5:180. doi: 10.3389/fimmu.2014.00180. eCollection 2014. Front Immunol. 2014. PMID: 24795723 Free PMC article. Review.
-
Natural and trained innate immunity against Mycobacterium tuberculosis.Immunobiology. 2020 May;225(3):151951. doi: 10.1016/j.imbio.2020.151951. Epub 2020 Apr 27. Immunobiology. 2020. PMID: 32423788 Review.
Cited by
-
Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma.Pharmaceutics. 2023 May 29;15(6):1611. doi: 10.3390/pharmaceutics15061611. Pharmaceutics. 2023. PMID: 37376061 Free PMC article. Review.
-
Host-directed therapy against mycobacterium tuberculosis infections with diabetes mellitus.Front Immunol. 2024 Jan 8;14:1305325. doi: 10.3389/fimmu.2023.1305325. eCollection 2023. Front Immunol. 2024. PMID: 38259491 Free PMC article. Review.
-
Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer.Pharmaceutics. 2024 Dec 11;16(12):1584. doi: 10.3390/pharmaceutics16121584. Pharmaceutics. 2024. PMID: 39771562 Free PMC article. Review.
-
Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment.Bone Res. 2023 Feb 27;11(1):11. doi: 10.1038/s41413-023-00246-z. Bone Res. 2023. PMID: 36849442 Free PMC article. Review.
-
Nanotechnology-driven therapies for neurodegenerative diseases: a comprehensive review.Ther Deliv. 2024;15(12):997-1024. doi: 10.1080/20415990.2024.2401307. Epub 2024 Sep 19. Ther Deliv. 2024. PMID: 39297726 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

